Inhibition of CILP2 Improves Glucose Metabolism and Mitochondrial Dysfunction in Sarcopenia via the Wnt Signalling Pathway
- PMID: 39385717
- PMCID: PMC11634484
- DOI: 10.1002/jcsm.13597
Inhibition of CILP2 Improves Glucose Metabolism and Mitochondrial Dysfunction in Sarcopenia via the Wnt Signalling Pathway
Abstract
Background: Skeletal muscle is the primary organ involved in insulin-mediated glucose metabolism. Elevated levels of CILP2 are a significant indicator of impaired glucose tolerance and are predominantly expressed in skeletal muscle. It remains unclear whether CILP2 contributes to age-related muscle atrophy through regulating the glucose homeostasis and insulin sensitivity.
Methods: Initially, the expression levels of CILP2 were assessed in elderly mice and patients with sarcopenia. Lentiviral vectors were used to induce either silencing or overexpression of CILP2 in C2C12 myoblast cells. The effects of CILP2 on proliferation, myogenic differentiation, insulin sensitivity and glucose uptake were evaluated using immunofluorescence, western blotting, real-time quantitative polymerase chain reaction, RNA sequencing, glucose uptake experiments, dual-luciferase reporter assays and co-immunoprecipitation (CO-IP). An adeno-associated virus-9 containing a muscle-specific promoter was injected into SAMP8 senile mice to observe the efficacy of CILP2 knockout.
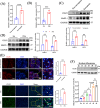
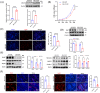
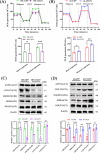
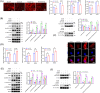
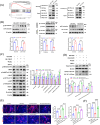
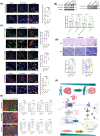
Results: We found that there was more CLIP2 expressed in the skeletal muscle of ageing mice (+1.1-fold, p < 0.01) and in patients with sarcopenia (+2.5-fold, p < 0.01) compared to the control group. Following the overexpression of CILP2, Ki67 (-65%, p < 0.01), PCNA (-32%, p < 0.05), MyoD1 (-89%, p < 0.001), MyoG (-31%, p < 0.05) and MyHC (-85%, p < 0.001), which indicate proliferation and differentiation potential, were significantly reduced. In contrast, MuRF-1 (+59%, p < 0.05), atrogin-1 (+43%, p < 0.05) and myostatin (+31%, p < 0.05), the markers of muscular atrophy, were significantly increased. Overexpression of CILP2 decreased insulin sensitivity, glucose uptake (-18%, p < 0.001), GLUT4 translocation to the membrane and the maximum respiratory capacity of mitochondria. Canonical Wnt signalling was identified through RNA sequencing as a potential pathway for CILP2 regulation in C2C12, and Wnt3a was confirmed as an interacting protein of CILP2 in the CO-IP assay. The addition of recombinant Wnt3a protein reversed the inhibitory effects on myogenesis and glucose metabolism caused by CILP2 overexpression. Conversely, CILP2 knockdown promoted myogenesis and glucose metabolism. CILP2 knockdown improved muscle atrophy in mice, characterized by significant increases in time to exhaustion (+42%, p < 0.001), grip strength (+19%, p < 0.01), muscle mass (+15%, p < 0.001) and mean muscle cross-sectional area (+37%, p < 0.01). CILP2 knockdown enhanced glycogen synthesis (+83%, p < 0.001) and the regeneration of oxidative and glycolytic muscle fibres in SAMP8 ageing mice via the Wnt/β-catenin signalling pathway.
Conclusions: Our results indicate that CILP2 interacts with Wnt3a to suppress the Wnt/β-catenin signalling pathway and its downstream cascade, leading to impaired insulin sensitivity and glucose metabolism in skeletal muscle. Targeting CILP2 inhibition could offer potential therapeutic benefits for sarcopenia.
Keywords: CILP2; Wnt/β‐catenin pathway; glucose metabolism; insulin resistance; sarcopenia.
© 2024 The Author(s). Journal of Cachexia, Sarcopenia and Muscle published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- 2020029HSJJ/Firestone Research Project of Fujian Provincial Hospital
- 2020J05270/Fujian Provincial Natural Science Foundation Projects
- 2022J011016/Fujian Provincial Natural Science Foundation Projects
- 2021J01376/Fujian Provincial Natural Science Foundation Projects
- 2020QNA009/Medical Innovation Project of Fujian Provincial Health Department
LinkOut - more resources
Full Text Sources
Miscellaneous

