Maternal oral probiotic use is associated with decreased breastmilk inflammatory markers, infant fecal microbiome variation, and altered recognition memory responses in infants-a pilot observational study
- PMID: 39385777
- PMCID: PMC11462058
- DOI: 10.3389/fnut.2024.1456111
Maternal oral probiotic use is associated with decreased breastmilk inflammatory markers, infant fecal microbiome variation, and altered recognition memory responses in infants-a pilot observational study
Abstract
Introduction: Early life gut microbiomes are important for brain and immune system development in animal models. Probiotic use has been proposed as a strategy to promote health via modulation of microbiomes. In this observational study, we explore if early life exposure to probiotics via the mother during pregnancy and lactation, is associated with decreased inflammation in breastmilk, maternal and infant microbiome variation, and altered infant neurodevelopmental features.
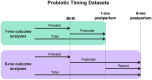
Methods: Exclusively breastfeeding mother-infant dyads were recruited as part of the "Mothers and Infants Linked for Healthy Growth (MILk) Study." Probiotic comparison groups were defined by exposure to maternal probiotics (NO/YES) and by timing of probiotic exposure (prenatal, postnatal, total). C-reactive protein (CRP) and IL-6 levels were determined in breastmilk by immunoassays, and microbiomes were characterized from 1-month milk and from 1- and 6-month infant feces by 16S rDNA sequencing. Infant brain function was profiled via electroencephalogram (EEG); we assessed recognition memory using event-related potential (ERP) responses to familiar and novel auditory (1 month) and visual (6 months) stimuli. Statistical comparisons of study outcomes between probiotic groups were performed using permutational analysis of variance (PERMANOVA) (microbiome) and linear models (all other study outcomes), including relevant covariables as indicated.
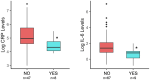
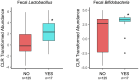
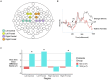
Results: We observed associations between probiotic exposure and lower breastmilk CRP and IL-6 levels, and infant gut microbiome variation at 1- and 6-months of age (including higher abundances of Bifidobacteria and Lactobacillus). In addition, maternal probiotic exposure was associated with differences in infant ERP features at 6-months of age. Specifically, infants who were exposed to postnatal maternal probiotics (between the 1- and 6-month study visits) via breastfeeding/breastmilk, had larger differential responses between familiar and novel visual stimuli with respect to the late slow wave component of the EEG, which may indicate greater memory updating potential. The milk of mothers of this subgroup of infants had lower IL-6 levels and infants had different 6-month fecal microbiomes as compared to those in the "NO" maternal probiotics group.
Discussion: These results support continued research into "Microbiota-Gut-Brain" connections during early life and the role of pre- and postnatal probiotics in mothers to promote healthy microbiome-associated outcomes in infants.
Keywords: Infant; Inflammation; breastmilk; event related potential; gut microbiome; neurodevelopment; probiotics.
Copyright © 2024 Gonia, Heisel, Miller, Haapala, Harnack, Georgieff, Fields, Knights, Jacobs, Seburg, Demerath, Gale and Swanson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu XN, et al. . Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice: commensal microbiota and stress response. J Physiol. (2004) 558:263–75. doi: 10.1113/jphysiol.2004.063388, PMID: - DOI - PMC - PubMed
-
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, et al. . Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci. (2010) 107:11971–5. doi: 10.1073/pnas.1002601107, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

