Semaglutide Versus Other Glucagon-Like Peptide-1 Agonists for Weight Loss in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis
- PMID: 39385875
- PMCID: PMC11463578
- DOI: 10.7759/cureus.69008
Semaglutide Versus Other Glucagon-Like Peptide-1 Agonists for Weight Loss in Type 2 Diabetes Patients: A Systematic Review and Meta-Analysis
Abstract
Obesity places patients at higher risk for numerous problems, including prediabetes, type 2 diabetes mellitus (T2DM), hypertension, metabolic syndrome, cardiovascular disease, and nonalcoholic fatty liver disease. Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) are antidiabetic drugs that have a recognized effect on weight loss. This systematic review analyzed semaglutide against alternative GLP-1 agonists in facilitating weight loss and evaluated their associated adverse events (AEs) in diabetic patients. A systematic search following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was performed using PubMed, Embase, and Cochrane Library for studies comparing semaglutide and other GLP-1 RAs for weight loss. A narrative synthesis and meta-analysis using SPSS program version 29 were performed to analyze the differences in weight loss between cohorts. Nine studies with 5,445 patients whose mean age was 60.01 years (55.5-70) and mean follow-up of 32.5 weeks (4-58.7) were included. The meta-analysis showed that semaglutide had a greater mean weight loss compared to liraglutide (-6.08, 95% confidence interval (Cl) = -8.40, -3.75) and dulaglutide (-2.85, 95% CI = -5.59, 0.11). Tirzepatide had a greater mean weight loss compared to semaglutide (-3.78, 95% CI = -5.52, -2.04). Common AEs included minor and moderate gastrointestinal events. In conclusion, GLP-1 RAs have shown efficacy in reducing body weight in T2DM patients. Semaglutide, liraglutide, dulaglutide, tirzepatide, and exenatide demonstrated mean weight loss reductions of 4.81 kg, 2.81 kg, 4.03 kg, 9.7 kg, and 1.9 kg, respectively, with high rates of minimal to moderate-severity AEs. Semaglutide demonstrated increased numerical weight loss compared to its comparators (dulaglutide, liraglutide, and exenatide). However, tirzepatide, a dual-agonist, produced greater weight loss compared to semaglutide. The paucity of comparative head-to-head trials prevents a definitive conclusion of the superiority of one GLP-1 RA over another.
Keywords: dulaglutide; exenatide; glp-1; liraglutide; semaglutide; tirzepatide; weight loss.
Copyright © 2024, Wen et al.
Conflict of interest statement
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following: Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work. Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work. Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Figures
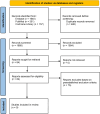
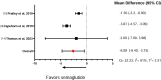



References
-
- Classification of obesity and assessment of obesity-related health risks. Aronne LJ. Obes Res. 2002;10 Suppl 2:105–115. - PubMed
-
- GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Meier JJ. Nat Rev Endocrinol. 2012;8:728–742. - PubMed
-
- The role of GLP‐1 receptor agonists as weight loss agents in patients with and without type 2 diabetes. Dar S, Tahrani AA, Piya MK. Pract Diabetes. 2015;32:297–300.
-
- Methodological index for non-randomized studies (minors): development and validation of a new instrument. Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. ANZ J Surg. 2003;73:712–716. - PubMed
-
- RoB 2: a revised tool for assessing risk of bias in randomised trials. Sterne JA, Savović J, Page MJ, et al. BMJ. 2019;366:0. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
