High accuracy meets high throughput for near full-length 16S ribosomal RNA amplicon sequencing on the Nanopore platform
- PMID: 39386005
- PMCID: PMC11462149
- DOI: 10.1093/pnasnexus/pgae411
High accuracy meets high throughput for near full-length 16S ribosomal RNA amplicon sequencing on the Nanopore platform
Abstract
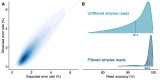
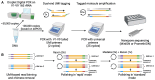
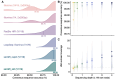
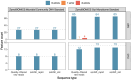
Small subunit (SSU) ribosomal RNA (rRNA) gene amplicon sequencing is a foundational method in microbial ecology. Currently, short-read platforms are commonly employed for high-throughput applications of SSU rRNA amplicon sequencing, but at the cost of poor taxonomic classification due to limited fragment lengths. The Oxford Nanopore Technologies (ONT) platform can sequence full-length SSU rRNA genes, but its lower raw-read accuracy has so-far limited accurate taxonomic classification and de novo feature generation. Here, we present a sequencing workflow, termed ssUMI, that combines unique molecular identifier (UMI)-based error correction with newer (R10.4+) ONT chemistry and sample barcoding to enable high throughput near full-length SSU rRNA (e.g. 16S rRNA) amplicon sequencing. The ssUMI workflow generated near full-length 16S rRNA consensus sequences with 99.99% mean accuracy using a minimum subread coverage of 3×, surpassing the accuracy of Illumina short reads. The consensus sequences generated with ssUMI were used to produce error-free de novo sequence features with no false positives with two microbial community standards. In contrast, Nanopore raw reads produced erroneous de novo sequence features, indicating that UMI-based error correction is currently necessary for high-accuracy microbial profiling with R10.4+ ONT sequencing chemistries. We showcase the cost-competitive scalability of the ssUMI workflow by sequencing 87 time-series wastewater samples and 27 human gut samples, obtaining quantitative ecological insights that were missed by short-read amplicon sequencing. ssUMI, therefore, enables accurate and low-cost full-length 16S rRNA amplicon sequencing on Nanopore, improving accessibility to high-resolution microbiome science.
Keywords: 16S rRNA; Nanopore; amplicon sequencing; long reads; microbiome.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures


References
-
- Giovannoni SJ, Britschgi TB, Moyer CL, Field KG. 1990. Genetic diversity in Sargasso sea bacterioplankton. Nature. 345:60–63. - PubMed
LinkOut - more resources
Full Text Sources

