Muscarinic receptor drug trihexyphenidyl can alter growth of mesenchymal glioblastoma in vivo
- PMID: 39386028
- PMCID: PMC11461351
- DOI: 10.3389/fphar.2024.1468920
Muscarinic receptor drug trihexyphenidyl can alter growth of mesenchymal glioblastoma in vivo
Abstract
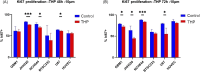
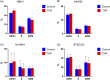
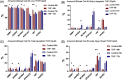
Glioblastoma (GBM) is the most commonly occurring and most aggressive primary brain tumor. Transcriptomics-based tumor subtype classification has established the mesenchymal lineage of GBM (MES-GBM) as cancers with particular aggressive behavior and high levels of therapy resistance. Previously it was show that Trihexyphenidyl (THP), a market approved M1 muscarinic receptor-targeting oral drug can suppress proliferation and survival of GBM stem cells from the classical transcriptomic subtype. In a series of in vitro experiments, this study confirms the therapeutic potential of THP, by effectively suppressing the growth, proliferation and survival of MES-GBM cells with limited effects on non-tumor cells. Transcriptomic profiling of treated cancer cells identified genes and associated metabolic signaling pathways as possible underlying molecular mechanisms responsible for THP-induced effects. In vivo trials of THP in immunocompromised mice carry orthotopic MES-GBMs showed moderate response to the drug. This study further highlights the potential of THP repurposing as an anti-cancer treatment regimen but mode of action and d optimal treatment procedures for in vivo regimens need to be investigated further.
Keywords: cystathionine beta-synthase; drug repurposing; glioblastoma; mesenchymal transformation; trihexyphenidyl.
Copyright © 2024 Du, Sanin, Shi, Huang, Nickel, Vargas-Toscano, Huo, Nickl-Jockschat, Dumitru, Hu, Duan, Sandalcioglu, Croner, Alcaniz, Walther, Berndt and Kahlert.
Conflict of interest statement
Authors JA and WW were employed by Experimental Pharmacology and Oncology Berlin-Buch GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

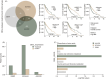
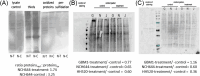
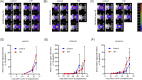
References
-
- Brocks D. R. (1999). Anticholinergic drugs used in Parkinson’s disease: an overlooked class of drugs from a pharmacokinetic perspective. J. Pharm. Pharm. Sci. Publ. Can. Soc. Pharm. Sci. Soc. Can. Sci. Pharm. 2, 39–46. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

