Immunocytochemistry assessment of vocal fold regeneration after cell-based implant in rabbits
- PMID: 39386157
- PMCID: PMC11462588
- DOI: 10.1002/lio2.70007
Immunocytochemistry assessment of vocal fold regeneration after cell-based implant in rabbits
Abstract
Objective: Cell-based outer vocal fold replacement (COVR) offers a potential treatment for severe vocal fold scarring or cancer reconstruction. Previous work in rabbits using human adipose-derived stem cells (ASC) in fibrin suggested that a hybrid structure emerged within 2 months, containing both implanted and host cells. This project uses immunocytochemistry to better define the phenotypic fate of implanted cells and features of the extracellular environment.
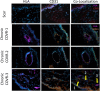
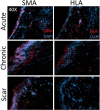
Methods: Immunocytochemistry was performed on sections collected from rabbits 2 months after COVR implantation or scar surgery. Cellular targets included human leukocyte antigen (HLA), CD31, and smooth muscle actin (SMA).
Results: HLA was present in all implanted sections and was used to identify human cells. In adjacent sections, HLA-positive cells were identified expressing CD31. SMA was not identified in the same cells as HLA. These markers were also present in injured vocal folds not receiving COVR. SMA protein content did not differ according to treatment.
Conclusions: Implanted human ASC persist in rabbit vocal folds. Some appear to express CD31, an endothelial marker. Smooth muscle actin, a marker of myofibroblast phenotype, was present in all sections regardless of treatment, and was not identified in hASC. Host cells also infiltrate the structure, producing a hybrid host-graft vocal fold.
Keywords: angiogenesis; cell‐based implant; human adipose derived stem cells; smooth muscle actin; vocal fold regeneration.
© 2024 The Author(s). Laryngoscope Investigative Otolaryngology published by Wiley Periodicals LLC on behalf of The Triological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Hansen JK, Thibeault SL. Current understanding and review of the literature: vocal fold scarring. J Voice. 2006;20(1):110‐120. - PubMed
-
- Nagubothu SR, Sugars RV, Tudzarovski N, et al. Mesenchymal stromal cells modulate tissue repair responses within the injured vocal fold. Laryngoscope. 2019;130(1):E21‐E29. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
