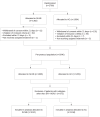
Axillary clearance and chemotherapy rates in ER+HER2- breast cancer: secondary analysis of the SENOMAC trial
- PMID: 39386258
- PMCID: PMC11460525
- DOI: 10.1016/j.lanepe.2024.101083
Axillary clearance and chemotherapy rates in ER+HER2- breast cancer: secondary analysis of the SENOMAC trial
Abstract
Background: Randomized trials have shown that axillary clearance (AC) can safely be omitted in patients with sentinel lymph node-positive breast cancer. At the same time, de-escalation of chemotherapy in postmenopausal patients with ER+HER2- breast cancer may depend on detailed axillary nodal stage. The aim of this pre-specified secondary analysis of the SENOMAC trial was to investigate whether the choice of axillary staging affected the proportion of patients receiving adjuvant chemotherapy, and recurrence-free survival (RFS).
Methods: Proportion receiving adjuvant chemotherapy was calculated according to AC or sentinel lymph node biopsy (SLNB) only, menopausal status, and region of inclusion, for 2168 patients with clinically node-negative ER+HER2- breast cancer and 1-2 sentinel lymph node macrometastases included in the SENOMAC trial.
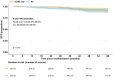
Findings: In premenopausal patients, 514 out of 615 patients (83.6%) received adjuvant chemotherapy with no significant difference between randomization arms. In postmenopausal patients, the proportion receiving chemotherapy varied considerably by region and country (36.0-82.4%). In Denmark, where 194 out of 539 postmenopausal patients (36.0%) received adjuvant chemotherapy, rates differed significantly between the AC and the SLNB only arm (41.3% vs 31.4%, p = 0.019). After a median follow-up of 44.88 months for Danish postmenopausal patients, no significant difference was seen in 5-year RFS, which was 91% (85.6%-96.6%) for the SLNB only and 90.9% (86.3%-95.6%) for the AC arm (p = 0.42).
Interpretation: When omitting axillary clearance, and thus reducing the risk of long-term arm morbidity, potential under-treatment of postmenopausal patients with ER+HER2- breast cancer may require the development of new predictive and imaging tools.
Funding: Swedish Research Council, Swedish Cancer Society, Nordic Cancer Union, Swedish Breast Cancer Association.
Keywords: Adjuvant treatment; Axillary staging; Breast cancer.
© 2024 The Author(s).
Conflict of interest statement
Dr. RO Bagge declares research grants from BMS, Endomagnetics Ltd, Skyline Dx and NeraCare GmbH as well as participation in advisory board for Amgen, BD/BARD, Bristol-Myers Squibb (BMS), Cansr.com, Merck Sharp & Dohme (MSD), Novartis, Roche and Sanofi Genzyme and stock or stock options in SATMEG Ventures AB. Thorsten Kuehn has payment or honoraria for presentations from MSD, Novartis, Lilly, Exact Sciences, Merit Medical, Sirius Medical and EndoMag and support for attending meetings from MSD, Lilly. All other authors declare no competing interests.
Figures
References
-
- LeVasseur N., Sun J., Fenton D., et al. Impact of the 21-gene recurrence score assay on the treatment of estrogen receptor-positive, HER2-negative, breast cancer patients with 1-3 positive nodes: a prospective clinical utility study. Clin Breast Cancer. 2022;22(1):e74–e79. doi: 10.1016/j.clbc.2021.09.004. - DOI - PubMed
-
- Piccart M., van 't Veer L.J., Poncet C., et al. 70-gene signature as an aid for treatment decisions in early breast cancer: updated results of the phase 3 randomised MINDACT trial with an exploratory analysis by age. Lancet Oncol. 2021;22:476–488. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous