This is a preprint.
Neural substrates for saccadic modulation of visual representations in mouse superior colliculus
- PMID: 39386422
- PMCID: PMC11463470
- DOI: 10.1101/2024.09.21.613770
Neural substrates for saccadic modulation of visual representations in mouse superior colliculus
Update in
-
Diverse and dynamic influences of saccades on visual representations in the mouse superior colliculus.Proc Natl Acad Sci U S A. 2025 Jul 22;122(29):e2425788122. doi: 10.1073/pnas.2425788122. Epub 2025 Jul 16. Proc Natl Acad Sci U S A. 2025. PMID: 40668831 Free PMC article.
Abstract
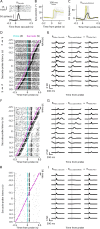
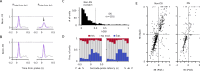
How do sensory systems account for stimuli generated by natural behavior? We addressed this question by examining how an ethologically relevant class of saccades modulates visual representations in the mouse superior colliculus (SC), a key region for sensorimotor integration. We quantified saccadic modulation by recording SC responses to visual probes presented at stochastic saccade-probe latencies. Saccades significantly impacted population representations of the probes, with early enhancement that began prior to saccades and pronounced suppression for several hundred milliseconds following saccades, independent of units' visual response properties or directional tuning. To determine the cause of saccadic modulation, we presented fictive saccades that simulated the visual experience during saccades without motor output. Some units exhibited similar modulation by fictive and real saccades, suggesting a sensory-driven origin of saccadic modulation, while others had dissimilar modulation, indicating a motor contribution. These findings advance our understanding of the neural basis of natural visual coding.
Conflict of interest statement
DECLARATION OF INTERESTS The authors declare no competing interests.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
