This is a preprint.
Versatile Cell Penetrating Peptide for Multimodal CRISPR Gene Editing in Primary Stem Cells
- PMID: 39386541
- PMCID: PMC11463527
- DOI: 10.1101/2024.09.23.614499
Versatile Cell Penetrating Peptide for Multimodal CRISPR Gene Editing in Primary Stem Cells
Abstract
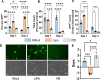
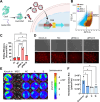
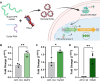
CRISPR gene editing offers unprecedented genomic and transcriptomic control for precise regulation of cell function and phenotype. However, delivering the necessary CRISPR components to therapeutically relevant cell types without cytotoxicity or unexpected side effects remains challenging. Viral vectors risk genomic integration and immunogenicity while non-viral delivery systems are challenging to adapt to different CRISPR cargos, and many are highly cytotoxic. The arginine-alanine-leucine-alanine (RALA) cell penetrating peptide is an amphiphilic peptide that self-assembles into nanoparticles through electrostatic interactions with negatively charged molecules before delivering them across the cell membrane. This system has been used to deliver DNAs, RNAs, and small anionic molecules to primary cells with lower cytotoxicity compared to alternative non-viral approaches. Given the low cytotoxicity, versatility, and competitive transfection rates of RALA, we aimed to establish this peptide as a new CRISPR delivery system in a wide range of molecular formats across different editing modalities. We report that RALA was able to effectively encapsulate and deliver CRISPR in DNA, RNA, and ribonucleic protein (RNP) formats to primary mesenchymal stem cells (MSCs). Comparisons between RALA and commercially available reagents revealed superior cell viability leading to higher numbers of transfected cells and the maintenance of cell proliferative capacity. We then used the RALA peptide for the knock-in and knock-out of reporter genes into the MSC genome as well as for the transcriptional activation of therapeutically relevant genes. In summary, we establish RALA as a powerful tool for safer and effective delivery of CRISPR machinery in multiple cargo formats for a wide range of gene editing strategies.
Keywords: CRISPR gene editing; Cell-penetrating peptide; mesenchymal stem cells; non-viral CRISPR delivery; regenerative medicine.
Conflict of interest statement
Competing Interests: The authors have no competing interests to share.
Figures



References
-
- Graham J., Werba L., Federico I., and Gonzalez-Fernandez T., “CRISPR Strategies for Stem Cell Engineering: A New Frontier in Musculoskeletal Regeneration,” Eur. Cell. Mater., vol. 46, pp. 91–118, Nov. 2023, doi: 10.22203/eCM.v046a05. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
