This is a preprint.
Modulation of Stemness and Differentiation Regulators by Valproic Acid in Medulloblastoma Neurospheres
- PMID: 39386542
- PMCID: PMC11463451
- DOI: 10.1101/2024.09.23.614476
Modulation of Stemness and Differentiation Regulators by Valproic Acid in Medulloblastoma Neurospheres
Update in
-
Modulation of Stemness and Differentiation Regulators by Valproic Acid in Medulloblastoma Neurospheres.Cells. 2025 Jan 7;14(2):72. doi: 10.3390/cells14020072. Cells. 2025. PMID: 39851500 Free PMC article.
Abstract
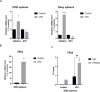
Changes in epigenetic processes such as histone acetylation are proposed as key events influencing cancer cell function and the initiation and progression of pediatric brain tumors. Valproic acid (VPA) is an antiepileptic drug that acts partially by inhibiting histone deacetylases (HDACs) and could be repurposed as an epigenetic anticancer therapy. Here, we show that VPA reduced medulloblastoma (MB) cell viability and led to cell cycle arrest. These effects were accompanied by enhanced H3K9 histone acetylation (H3K9ac) and decreased expression of the MYC oncogene. VPA impaired the expansion of MB neurospheres enriched in stemness markers and reduced MYC while increasing TP53 expression in these neurospheres. In addition, VPA induced morphological changes consistent with neuronal differentiation and the increased expression of differentiation marker genes TUBB3 and ENO2. The expression of stemness genes SOX2, NES, and PRTG was differentially affected by VPA in MB cells with different TP53 status. VPA increased H3K9 occupancy of the promoter region of TP53. Among the genes regulated by VPA, the stemness regulators MYC and NES showed an association with patient survival in specific MB subgroups. Our results indicate that VPA may exert antitumor effects in MB by influencing histone acetylation, which may result in the modulation of stemness, neuronal differentiation, and the expression of genes associated with patient prognosis in specific molecular subgroups. Importantly, the actions of VPA in MB cells and neurospheres include a reduction in the expression of MYC and an increase in TP53.
Keywords: MYC; TP53; differentiation; medulloblastoma; stemness; valproic acid.
Conflict of interest statement
Conflicts of Interest: The authors declare no conflicts of interest related to the contents of this manuscript.
Figures





References
-
- Schüller U.; Heine V.M.; Mao J.; Kho A.T.; Dillon A.K.; Han Y.G.; Huillard E.; Sun T.; Ligon A.H.; Qian Y.; et al. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell 2008, 14, 123–134. 10.1016/j.ccr.2008.07.005. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
