This is a preprint.
Expression-based selection identifies a microglia-tropic AAV capsid for direct and CSF routes of administration in mice
- PMID: 39386560
- PMCID: PMC11463440
- DOI: 10.1101/2024.09.25.614546
Expression-based selection identifies a microglia-tropic AAV capsid for direct and CSF routes of administration in mice
Abstract
Microglia are critical innate immune cells of the brain. In vivo targeting of microglia using gene-delivery systems is crucial for studying brain physiology and developing gene therapies for neurodegenerative diseases and other brain disorders such as NeuroAIDS. Historically, microglia have been extremely resistant to transduction by viral vectors, including adeno-associated virus (AAV) vectors. Recently, there has been some progress demonstrating the feasibility and potential of using AAV to transduce microglia after direct intraparenchymal vector injection. Data suggests that combining specific AAV capsids with microglia-specific gene expression cassettes to reduce neuron off-targeting will be key. However, no groups have developed AAV capsids for microglia transduction after intracerebroventricular (ICV) injection. The ICV route of administration has advantages such as increased brain biodistribution while avoiding issues related to systemic injection. Here, we performed an in vivo selection using an AAV peptide display library that enables recovery of capsids that mediate transgene expression in microglia. Using this approach, we identified a capsid, MC5, which mediated enhanced transduction of microglia after ICV injection compared to AAV9. Furthermore, MC5 enhanced both the efficiency (85%) and specificity (93%) of transduction compared to a recently described evolved AAV9 capsid for microglia targeting after direct injection into the brain parenchyma. Exploration of the use of MC5 in a mouse models of Alzheimer's disease revealed transduced microglia surrounding and within plaques. Overall, our results demonstrate that the MC5 capsid is a useful gene transfer tool to target microglia in vivo by direct and ICV routes of administration.
Conflict of interest statement
Competing interests: C.A.M. has a financial interest in Sphere Gene Therapeutics, Inc., Chameleon Biosciences, Inc., and Skylark Bio, Inc., companies developing gene therapy platforms. C.A.M.’s interests were reviewed and are managed by MGH and Mass General Brigham in accordance with their conflict-of-interest policies. C.A.M., M.C.S., K.S.H., and P.E. have filed a patent application with claims involving the MC5 capsid.
Figures


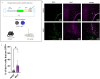
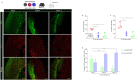
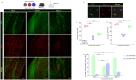

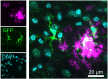
References
-
- Lin R, Zhou Y, Yan T, Wang R, Li H, Wu Z, Zhang X, Zhou X, Zhao F, Zhang L, et al. Directed evolution of adeno-associated virus for efficient gene delivery to microglia. Nat Methods. 2022;19(8):976–85. - PubMed
-
- Lek A, Wong B, Keeler A, Blackwood M, Ma K, Huang S, Sylvia K, Batista AR, Artinian R, Kokoski D, et al. Unexpected Death of a Duchenne Muscular Dystrophy Patient in an N-of-1 Trial of rAAV9-delivered CRISPR-transactivator. medRxiv. 2023:2023.05.16.23289881.
-
- Wilson JM, Flotte TR. Moving Forward After Two Deaths in a Gene Therapy Trial of Myotubular Myopathy. Hum Gene Ther. 2020;31(13-14):695–6. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
