This is a preprint.
Potentiation of active locomotor state by spinal-projecting serotonergic neurons
- PMID: 39386605
- PMCID: PMC11463418
- DOI: 10.1101/2024.09.26.615260
Potentiation of active locomotor state by spinal-projecting serotonergic neurons
Abstract
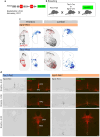
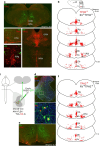
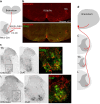
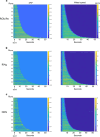
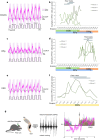
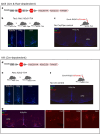
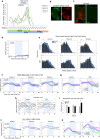
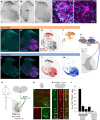
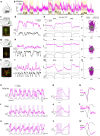
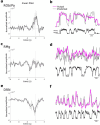
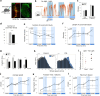
Animals produce diverse motor actions that enable expression of context-appropriate behaviors. Neuromodulators facilitate behavioral flexibility by altering the temporal dynamics and output of neural circuits. Discrete populations of serotonergic (5-HT) neurons target circuits in the brainstem and spinal cord, but their role in the control of motor behavior is unclear. Here we define the pre- and post-synaptic organization of the spinal-projecting serotonergic system and define a role in locomotor control. We show that while forebrain-targeting 5-HT neurons decrease their activity during locomotion, subpopulations of spinal projecting neurons increase their activity in a context-dependent manner. Optogenetic activation of ventrally projecting 5-HT neurons does not trigger initiation of movement, but rather enhances the speed and duration of ongoing locomotion over extended time scales. These findings indicate that the descending serotonergic system potentiates locomotor output and demonstrate a role for serotonergic neurons in modulating the temporal dynamics of motor circuits.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
