This is a preprint.
Multimodal Spatial Profiling Reveals Immune Suppression and Microenvironment Remodeling in Fallopian Tube Precursors to High-Grade Serous Ovarian Carcinoma
- PMID: 39386723
- PMCID: PMC11463462
- DOI: 10.1101/2024.09.25.615007
Multimodal Spatial Profiling Reveals Immune Suppression and Microenvironment Remodeling in Fallopian Tube Precursors to High-Grade Serous Ovarian Carcinoma
Update in
-
Multimodal Spatial Profiling Reveals Immune Suppression and Microenvironment Remodeling in Fallopian Tube Precursors to High-Grade Serous Ovarian Carcinoma.Cancer Discov. 2025 Jun 3;15(6):1180-1202. doi: 10.1158/2159-8290.CD-24-1366. Cancer Discov. 2025. PMID: 39704522 Free PMC article.
Abstract
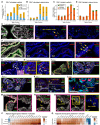
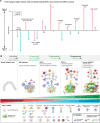
High-Grade Serous Ovarian Cancer (HGSOC) originates from fallopian tube (FT) precursors. However, the molecular changes that occur as precancerous lesions progress to HGSOC are not well understood. To address this, we integrated high-plex imaging and spatial transcriptomics to analyze human tissue samples at different stages of HGSOC development, including p53 signatures, serous tubal intraepithelial carcinomas (STIC), and invasive HGSOC. Our findings reveal immune modulating mechanisms within precursor epithelium, characterized by chromosomal instability, persistent interferon (IFN) signaling, and dysregulated innate and adaptive immunity. FT precursors display elevated expression of MHC-class I, including HLA-E, and IFN-stimulated genes, typically linked to later-stage tumorigenesis. These molecular alterations coincide with progressive shifts in the tumor microenvironment, transitioning from immune surveillance in early STICs to immune suppression in advanced STICs and cancer. These insights identify potential biomarkers and therapeutic targets for HGSOC interception and clarify the molecular transitions from precancer to cancer.
Keywords: HLA-A; HLA-E; High Grade Serous Ovarian Carcinoma; Natural Killer cells; Ovarian cancer; Serous Tubal Intraepithelial Carcinoma; antigen presentation; cancer progression; fallopian tube; homologous recombinant deficient tumor; innate immune system; multi-plex imaging; p53 signatures; preneoplasia; single cell; spatial transcriptomics; tumor immune interaction; tumor microenvironment.
Conflict of interest statement
DECLARATION OF INTERESTS PKS is a co-founder and member of the BOD of Glencoe Software and member of the SAB for RareCyte, NanoString, and Montai Health; he holds equity in Glencoe and RareCyte. PKS is a consultant for Merck. RD is a member of the SAB for Repare Therapeutics and is a consultant for Light Horse Therapeutics and Abbvie. The other authors declare no outside interests.
Figures





References
-
- Bray F. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 74, 229–263 (2024). - PubMed
-
- Lee Y. et al. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J. Pathol. 211, 26–35 (2007). - PubMed
-
- Medeiros F. et al. The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome. Am. J. Surg. Pathol. 30, 230–236 (2006). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
