Palladium and copper co-catalyzed chloro-arylation of gem-difluorostyrenes - use of a nitrite additive to suppress β-F elimination
- PMID: 39386912
- PMCID: PMC11456958
- DOI: 10.1039/d4sc04939j
Palladium and copper co-catalyzed chloro-arylation of gem-difluorostyrenes - use of a nitrite additive to suppress β-F elimination
Abstract
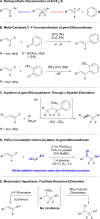
The installation of fluorine and fluorinated functional groups in organic molecules perturbs the physicochemical properties of those molecules and enables the development of new therapeutics, agrichemicals, biological probes and materials. However, current synthetic methodologies cannot access some fluorinated functional groups and fluorinated scaffolds. One such group, the gem-difluorobenzyl motif, might be convergently synthesized by reacting a nucleophilic aryl precursor and an electrophilic gem-difluoroalkene. Previous attempts have relied on forming unstable anionic or organometallic intermediates that rapidly decompose through a β-F elimination process to deliver monofluorovinyl products. In contrast, we report a fluorine-retentive palladium and copper co-catalyzed chloro-arylation of gem-difluorostyrenes that takes advantage of a nitrite (NO2 -) additive to avoid the favorable β-F elimination pathway that forms monofluorinated products, instead delivering difluorinated products.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures


Similar articles
-
Acid-Catalyzed Hydrothiolation of gem-Difluorostyrenes to Access α,α-Difluoroalkylthioethers.J Org Chem. 2021 Feb 5;86(3):2297-2311. doi: 10.1021/acs.joc.0c02440. Epub 2021 Jan 20. J Org Chem. 2021. PMID: 33471529 Free PMC article.
-
Arylation of gem-difluoroalkenes using a Pd/Cu Co-catalytic system that avoids β-fluoride elimination.Chem Sci. 2020 Nov 25;12(4):1363-1367. doi: 10.1039/d0sc05192f. Chem Sci. 2020. PMID: 34163899 Free PMC article.
-
Peroxide-Initiated Hydrophosphinylation of gem-Difluoroalkenes.J Org Chem. 2023 Oct 6;88(19):14012-14021. doi: 10.1021/acs.joc.3c01562. Epub 2023 Sep 22. J Org Chem. 2023. PMID: 37738112 Free PMC article.
-
The organometallic fluorine chemistry of palladium and rhodium: studies toward aromatic fluorination.Acc Chem Res. 2010 Jan 19;43(1):160-71. doi: 10.1021/ar9001763. Acc Chem Res. 2010. PMID: 19788304 Review.
-
Electrophilic halogenation-reductive elimination chemistry of organopalladium and -platinum complexes.Acc Chem Res. 2015 Feb 17;48(2):238-47. doi: 10.1021/ar500325x. Epub 2015 Jan 20. Acc Chem Res. 2015. PMID: 25602260 Review.
Cited by
-
Palladium-Catalyzed Dual Csp2─Csp3 Bond Formation: A Versatile Platform for the Synthesis of Benzo-Fused Heterocycles.Adv Sci (Weinh). 2025 Jul;12(25):e2500897. doi: 10.1002/advs.202500897. Epub 2025 Apr 28. Adv Sci (Weinh). 2025. PMID: 40289651 Free PMC article.
References
-
- Umemoto T. Singh R. P. Xu Y. Saito N. Discovery of 4-Tert-Butyl-2,6-Dimethylphenylsulfur Trifluoride as a Deoxofluorinating Agent with High Thermal Stability as Well as Unusual Resistance to Aqueous Hydrolysis, and Its Diverse Fluorination Capabilities Including Deoxofluoro-Arylsulfinylation. J. Am. Chem. Soc. 2010;132(51):18199–18205. doi: 10.1021/ja106343h. doi: 10.1021/ja106343h. - DOI - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

