A human model of Buruli ulcer: Provisional protocol for a Mycobacterium ulcerans controlled human infection study
- PMID: 39386965
- PMCID: PMC11462124
- DOI: 10.12688/wellcomeopenres.22719.2
A human model of Buruli ulcer: Provisional protocol for a Mycobacterium ulcerans controlled human infection study
Abstract
Critical knowledge gaps have impeded progress towards reducing the global burden of disease due to Mycobacterium ulcerans, the cause of the neglected tropical disease Buruli ulcer (BU). Development of a controlled human infection model of BU has been proposed as an experimental platform to explore host-pathogen interactions and evaluate tools for prevention, diagnosis, and treatment. We have previously introduced the use case for a new human model and identified M. ulcerans JKD8049 as a suitable challenge strain. Here, we present a provisional protocol for an initial study, for transparent peer review during the earliest stages of protocol development. Following simultaneous scientific peer review and community/stakeholder consultation of this provisional protocol, we aim to present a refined protocol for institutional review board (IRB) evaluation.
Keywords: Bairnsdale ulcer; Buruli ulcer; M. ulcerans; Mycobacterium ulcerans; controlled human infection model.
Plain language summary
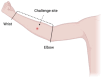
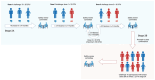
This paper describes a provisional clinical protocol for the pilot human challenge model of Mycobacterium ulcerans infection, which causes the skin disease 'Buruli ulcer' (BU). BU is typically painless and begins as a small area of redness or swelling, and is curable with antibiotics. If the diagnosis is delayed, it can result in large ulceration and disability. Side effects from antibiotics are common but rarely severe; nevertheless, preventative strategies, such as vaccination, are urgently needed. The overarching project, known as 'MuCHIM', aims to establish a safe and acceptable controlled human challenge model (CHIM) of this disease in healthy volunteers in Melbourne, Australia. This pilot protocol primarily aims to establish that it is safe and acceptable to participants, and secondarily to confirm successful establishment of infection and the infection rate amongst participants. We also aim to test less invasive diagnostic tests, assess immune responses to infection, to understand changes in the human microbiome during the trial, and explore microbiological characteristics of M. ulcerans infection. If this pilot is successful, we hope to test vaccines and other therapeutics using this model, which could blunt or reduce the rising incidence of this disease in Australia, while further informing vaccine development research.
Copyright: © 2024 Muhi S et al.
Conflict of interest statement
No competing interests were disclosed.
Figures
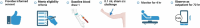











References
-
- Locally transmitted Buruli ulcer cases identified on the NSW south coast.2023; [cited 2024 Feb 28]. Reference Source
-
- Health advisory: Buruli ulcer is spreading.2023; [cited 2024 Feb 28]. Reference Source
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources

