Immunoregulatory Macrophages Modify Local Pulmonary Immunity and Ameliorate Hypoxic Pulmonary Hypertension
- PMID: 39387119
- PMCID: PMC11697987
- DOI: 10.1161/ATVBAHA.124.321264
Immunoregulatory Macrophages Modify Local Pulmonary Immunity and Ameliorate Hypoxic Pulmonary Hypertension
Abstract
Background: Macrophages play a significant role in the onset and progression of vascular disease in pulmonary hypertension, and cell-based immunotherapies aimed at treating vascular remodeling are lacking. We aimed to evaluate the effect of pulmonary administration of macrophages modified to have an anti-inflammatory/proresolving phenotype in attenuating early pulmonary inflammation and progression of experimentally induced pulmonary hypertension.
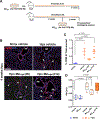
Methods: Mouse bone marrow-derived macrophages were polarized in vitro to a regulatory (M2reg) phenotype. M2reg profile and anti-inflammatory capacity were assessed in vitro upon lipopolysaccharide/IFNγ (interferon-γ) restimulation, before their administration to 8- to 12-week-old mice. M2reg protective effect was evaluated at early (2-4 days) and late (4 weeks) time points during hypoxia (8.5% O2) exposure. Levels of inflammatory markers were quantified in alveolar macrophages and whole lung, while pulmonary hypertension development was ascertained by right ventricular systolic pressure (RVSP) and right ventricular hypertrophy measurements. Bronchoalveolar lavage from M2reg-transplanted hypoxic mice was collected and its inflammatory potential evaluated on naive bone marrow-derived macrophages.
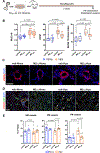
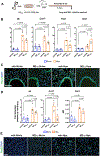
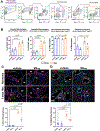
Results: M2reg macrophages expressing Tgfβ, Il10, and Cd206 demonstrated a stable anti-inflammatory phenotype in vitro, by downregulating the induction of proinflammatory cytokines and surface molecules (Cd86, Il6, and Tnfα) upon a subsequent proinflammatory stimulus. A single dose of M2regs attenuated hypoxic monocytic recruitment and perivascular inflammation. Early hypoxic lung and alveolar macrophage inflammation leading to pulmonary hypertension development was significantly reduced, and, importantly, M2regs attenuated right ventricular hypertrophy, right ventricular systolic pressure, and vascular remodeling at 4 weeks post-treatment.
Conclusions: Adoptive transfer of M2regs halts the recruitment of monocytes and modifies the hypoxic lung microenvironment, potentially changing the immunoreactivity of recruited macrophages and restoring normal immune functionality of the lung. These findings provide new mechanistic insights into the diverse role of macrophage phenotype on lung vascular homeostasis that can be explored as novel therapeutic targets.
Keywords: adoptive transfer; hypoxia; macrophage activation; pulmonary arterial hypertension; vascular remodeling.
Conflict of interest statement
None.
Figures


Update of
-
Immunoregulatory macrophages modify local pulmonary immunity and ameliorate hypoxic-pulmonary hypertension.bioRxiv [Preprint]. 2023 Aug 2:2023.07.31.551394. doi: 10.1101/2023.07.31.551394. bioRxiv. 2023. Update in: Arterioscler Thromb Vasc Biol. 2024 Dec;44(12):e288-e303. doi: 10.1161/ATVBAHA.124.321264. PMID: 37577587 Free PMC article. Updated. Preprint.
References
-
- Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:897–908. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

