Dopamine Transporter and CYP2D6 Gene Relationships with Attention-Deficit/Hyperactivity Disorder Treatment Response in the Methylphenidate and Atomoxetine Crossover Study
- PMID: 39387268
- PMCID: PMC11807865
- DOI: 10.1089/cap.2024.0069
Dopamine Transporter and CYP2D6 Gene Relationships with Attention-Deficit/Hyperactivity Disorder Treatment Response in the Methylphenidate and Atomoxetine Crossover Study
Abstract
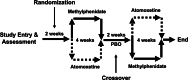
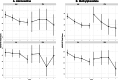
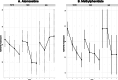
Background: Few biological or clinical predictors guide medication selection and/or dosing for attention-deficit/hyperactivity disorder (ADHD). Accumulating data suggest that genetic factors may contribute to clinically relevant pharmacodynamic (e.g., dopamine transporter-SLC6A3 also commonly known as DAT1) or pharmacokinetic (e.g., the drug metabolizing enzyme Cytochrome P450 2D6 CYP2D6) effects of methylphenidate (stimulant) and atomoxetine (non-stimulant), which are commonly prescribed medications. This is the first study of youth with ADHD exposed to both medications examining the clinical relevance of genetic variation on treatment response. Methods: Genetic variations in DAT1 and CYP2D6 were examined to determine how they modified time relationships with changes in ADHD symptoms over a 4-week period in 199 youth participating in a double-blind crossover study following a stepped titration dose optimization protocol. Results: Our results identified trends in the modification effect from CYP2D6 phenotype and the time-response relationship between ADHD total symptoms for both medications (atomoxetine [ATX]: p = 0.058, Methylphenidate [MPH]: p = 0.044). There was also a trend for the DAT1 3' untranslated region (UTR) variable number of tandem repeat (VNTR) genotype to modify dose relationships with ADHD-RS total scores for atomoxetine (p = 0.029). Participants with DAT1 9/10 repeat genotypes had a more rapid dose-response to ATX compared to 10/10, while those with 9/9 genotypes did not respond as doses were increased. Regardless of genotype, ADHD symptoms and doses were similar across CYP2D6 metabolizer groups after 4 weeks of treatment. Conclusions: Most children with ADHD who were CYP2D6 normal metabolizers or had DAT1 10/10 or 9/10 genotypes responded well to both medications. While we observed some statistically significant effects of CYP2D6 and DAT1 with treatment response over time, our data indicate that genotyping for clinical purposes may have limited utility to guide treatment decisions for ATX or MPH because both medications were generally effective in the studied cohort after 3 weeks of titration to higher doses. The potential DAT1 association with ATX treatment is a novel finding, consistent with prior reports suggesting an association of the DAT1 in 9/9 genotypes with lower responsive rates to treatment at low and moderate doses.
Keywords: CYP2D6; atomoxetine; dopamine transporter; methylphenidate; pharmacogenetics.
Figures
References
-
- Anonymous n.d . FDA product information summarized at Drugs@FDA: FDA-approved drugs. https://www.fda.gov/drugs
-
- Barkley RA, Smith KM, Fischer M. ADHD risk genes involved in dopamine signaling and metabolism are associated with reduced estimated life expectancy at young adult follow-up in hyperactive and control children. Am J Med Genet B Neuropsychiatr Genet 2019;180(3):175–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical