Systematic analysis based on bioinformatics and experimental validation identifies Alox5 as a novel therapeutic target of quercetin for sepsis
- PMID: 39387547
- PMCID: PMC11469444
- DOI: 10.1080/07853890.2024.2411015
Systematic analysis based on bioinformatics and experimental validation identifies Alox5 as a novel therapeutic target of quercetin for sepsis
Abstract
Purpose: This study investigated the molecular mechanism of quercetin in the treatment of sepsis using network pharmacological prediction and experimentation.
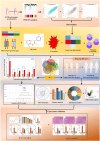
Methods: Hub genes were identified by intersecting the differentially expressed genes (DEGs) of the GSE131761 and GSE9960 databases with genes from the hub modules of Weighted Gene Co-Expression Network Analysis (WGCNA), targets of quercetin, and ferroptosis. Subsequently, in order to determine the functional characteristics and molecular link of hub gene obtained above, we redetermined the hub-DEGs in GSE131761 according to high or low hub gene expression. Afterward, the main pathways of enrichment analysis were validated using these hub-DEGs. Finally, an experiment was conducted to validate the findings.
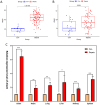
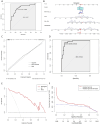
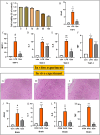
Results: By intersecting 1415 DEGs in GSE131761, 543 DEGs in GSE9960, 5784 key modular genes, 470 ferroptosis-related genes, and 154 quercetin-related genes, we obtained one quercetin-related gene, Alox5. Subsequently, 340 hub-DEGs were further validated according to high or low Alox5 expression. The results of the enrichment analysis revealed that hub-DEGs were mainly associated with inflammation and the immune response. Immune infiltration analysis showed that higher expression of Alox5 was related to macrophage infiltration and could be a predictor of diagnosis in patients with sepsis. The expression pattern of Alox5 was then depicted and the upregulation of Alox5 in the vital organs of septic mice was further demonstrated. In vitro and in vivo experiments showed that upregulation of Alox5 and inflammation-related cytokines induced by sepsis could be inhibited by quercetin (p < 0.05).
Conclusions: Alox5 may be involved in the occurrence and development of multi-organ functional disturbances in sepsis and is a reliable target of quercetin against sepsis.
Keywords: Alox5; inflammation; peripheral blood mononuclear cells; quercetin; sepsis.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures






References
-
- Abe T, Yamakawa K, Ogura H, et al. . Epidemiology of sepsis and septic shock in intensive care units between sepsis-2 and sepsis-3 populations: sepsis prognostication in intensive care unit and emergency room (SPICE-ICU). J Intensive Care. 2020;8(1):44. doi: 10.1186/s40560-020-00465-0. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
