Lipophilic bisphosphonates reduced cyst burden and ameliorated hyperactivity of mice chronically infected with Toxoplasma gondii
- PMID: 39387586
- PMCID: PMC11558998
- DOI: 10.1128/mbio.01756-24
Lipophilic bisphosphonates reduced cyst burden and ameliorated hyperactivity of mice chronically infected with Toxoplasma gondii
Abstract
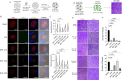
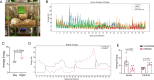
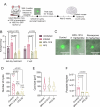
The current treatments for toxoplasmosis are only active against fast-growing tachyzoites, present in acute infections, with little effect on slow-growing bradyzoites within tissue cysts, present in latent chronic infections. The mitochondrion of Toxoplasma gondii is essential for its survival, and one of the major anti-parasitic drugs, atovaquone, inhibits the mitochondrial electron transport chain at the coenzyme Q:cytochrome c oxidoreductase site. Coenzyme Q (also known as ubiquinone [UQ]) consists of a quinone head and a lipophilic, isoprenoid tail that anchors UQ to membranes. The synthesis of the isoprenoid unit is essential for cell growth and is inhibited by lipophilic bisphosphonates, which inhibit the parasite growth. In this work, we investigated the effect of lipophilic bisphosphonates on the chronic stages of T. gondii. We discovered that three lipophilic bisphosphonates (BPH-1218, BPH-1236, and BPH-1238), effective for the acute infection, were also effective in controlling the development of chronic stages. We showed effectiveness by testing them against in vitro cysts and in vivo derived tissue cysts and, most importantly, these compounds reduced the cyst burden in the brains of chronically infected mice. We monitored the activity of infected mice non-invasively and continuously with a novel device termed the CageDot. A decrease in activity accompanied the acute phase, but mice recovered to normal activity and showed signs of hyperactivity when the chronic infection was established. Moreover, treatment with atovaquone or BPH-1218 ameliorated the hyperactivity observed during the chronic infection.IMPORTANCETreatment for toxoplasmosis is challenged by a lack of effective drugs to eradicate the chronic stages. Most of the drugs currently used are poorly distributed to the central nervous system, and they trigger allergic reactions in a large number of patients. There is a compelling need for safe and effective treatments for toxoplasmosis. Bisphosphonates (BPs) are analogs of inorganic pyrophosphate and are used for the treatment of bone disorders. BPs target the isoprenoid pathway and are effective against several experimental parasitic infections. Some lipophilic BPs can specifically inhibit the mitochondrial activity of Toxoplasma gondii by interfering with the mechanism by which ubiquinone is inserted into the inner mitochondrial membrane. In this work, we present the effect of three lipophilic BPs against T. gondii chronic stages. We also present a new strategy for the monitoring of animal activity during disease and treatment that is non-invasive and continuous.
Keywords: Toxoplasma gondii; bisphosphonate; bradyzoite; hyperactivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
- R21 AI147661/AI/NIAID NIH HHS/United States
- R01 HL172291/HL/NHLBI NIH HHS/United States
- 2312974/National Science Foundation (NSF)
- 2324389/National Science Foundation (NSF)
- 2019311/National Science Foundation (NSF)
- 1940864/National Science Foundation (NSF)
- T32 AI060546/AI/NIAID NIH HHS/United States
- R01 AI169846/AI/NIAID NIH HHS/United States
- R01HL17229/HHS | National Institutes of Health (NIH)
- Harriet A. Harlin Professorship
- R01AI169846/HHS | National Institutes of Health (NIH)
- EE0009026/U.S. Department of Defense (DOD)
- R21AI147661/HHS | National Institutes of Health (NIH)
- FA8571-21-C-0020/U.S. Department of Defense (DOD)
- T32AI060546/HHS | National Institutes of Health (NIH)
- University of Illinois Foundation
LinkOut - more resources
Full Text Sources

