Novel motif associated with carbon catabolite repression in two major Gram-positive pathogen virulence regulatory proteins
- PMID: 39387597
- PMCID: PMC11537053
- DOI: 10.1128/spectrum.00485-24
Novel motif associated with carbon catabolite repression in two major Gram-positive pathogen virulence regulatory proteins
Abstract
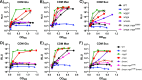
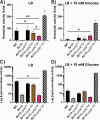
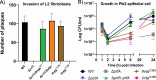
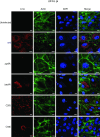
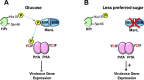
Carbon catabolite repression (CCR) is a widely conserved regulatory process that ensures enzymes and transporters of less-preferred carbohydrates are transcriptionally repressed in the presence of a preferred carbohydrate. This phenomenon can be regulated via a CcpA-dependent or CcpA-independent mechanism. The CcpA-independent mechanism typically requires a transcriptional regulator harboring a phosphotransferase regulatory domain (PRD) that interacts with phospho
Keywords: CCR; Listeria monocytogenes; Mga; PrfA; Streptococcus; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Henstra SA, Duurkens RH, Robillard GT. 2000. Multiple phosphorylation events regulate the activity of the mannitol transcriptional regulator MtlR of the Bacillus stearothermophilus phosphoenolpyruvate-dependent mannitol phosphotransferase system. J Biol Chem 275:7037–7044. doi:10.1074/jbc.275.10.7037 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

