Taming Tethered Nitreniums for Alkene Functionalization Reactions
- PMID: 39387609
- PMCID: PMC11827887
- DOI: 10.1021/acs.joc.4c01886
Taming Tethered Nitreniums for Alkene Functionalization Reactions
Abstract
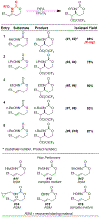
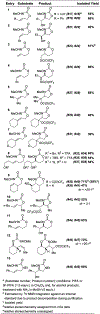
We present the first examples of amino-trifluoroacetoxylations of alkenes using N-alkoxy carbamate tethers. Hypervalent iodine oxidants mediate this transformation, providing a "green" alternative to existing intramolecular amino-hydroxylation protocols which use toxic metals such as osmium. In all cases examined, the reaction is regioselective and stereospecific, with the geometry of the starting alkene controlling the diastereomeric outcome. By analogy to prior art and from our own observations, we posit that a transient nitrenium species serves as a key intermediate.
Figures




References
-
- Luo Y; Zhang X; Xia Y, Recent advances in transition-metal catalyzed nitrene transfer reactions with carbamates. Chin. Chem. Lett 2024, 35, 108778.
-
- Müller P; Fruit C, Enantioselective Catalytic Aziridinations and Asymmetric Nitrene Insertions into CH Bonds. Chem. Rev 2003, 103, 2905–2920. - PubMed
-
- Trowbridge A; Walton SM; Gaunt MJ, New Strategies for the Transition-Metal Catalyzed Synthesis of Aliphatic Amines. Chem. Rev 2020, 120, 2613–2692. - PubMed
-
- Noda H; Tang X; Shibasaki M, Catalyst-Controlled Chemoselective Nitrene Transfers. Helv. Chim. Acta 2021, 104, e2100140.
Grants and funding
LinkOut - more resources
Full Text Sources

