Myosin-5a facilitates stress granule formation by interacting with G3BP1
- PMID: 39387926
- PMCID: PMC11467138
- DOI: 10.1007/s00018-024-05468-w
Myosin-5a facilitates stress granule formation by interacting with G3BP1
Abstract
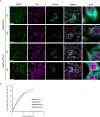
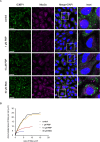
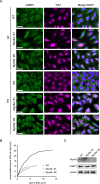
Stress granules (SGs) are non-membranous organelles composed of mRNA and proteins that assemble in the cytosol when the cell is under stress. Although the composition of mammalian SGs is both cell-type and stress-dependent, they consistently contain core components, such as Ras GTPase activating protein SH3 domain binding protein 1 (G3BP1). Upon stress, living cells rapidly assemble micrometric SGs, sometimes within a few minutes, suggesting that SG components may be actively transported by the microtubule and/or actin cytoskeleton. Indeed, SG assembly has been shown to depend on the microtubule cytoskeleton and the associated motor proteins. However, the role of the actin cytoskeleton and associated myosin motor proteins remains controversial. Here, we identified G3BP1 as a novel binding protein of unconventional myosin-5a (Myo5a). G3BP1 uses its C-terminal RNA-binding domain to interact with the middle portion of Myo5a tail domain (Myo5a-MTD). Suppressing Myo5a function in mammalian cells, either by overexpressing Myo5a-MTD, eliminating Myo5a gene expression, or treatment with myosin-5 inhibitor, inhibits the arsenite-induced formation of both small and large SGs. This is different from the effect of microtubule disruption, which abolishes the formation of large SGs but enhances the formation of small SGs under stress conditions. We therefore propose that, under stress conditions, Myo5a facilitates the formation of SGs at an earlier stage than the microtubule-dependent process.
Keywords: Actin; Arsenite; Intrinsically disordered region; Microtubule; Motor protein.
© 2024. The Author(s).
Conflict of interest statement
The authors have no relevant financial or non-financial interests to disclose.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

