Real-world effectiveness and safety of trastuzumab-deruxtecan in Japanese patients with HER2-positive advanced gastric cancer (EN-DEAVOR study)
- PMID: 39387986
- PMCID: PMC11706843
- DOI: 10.1007/s10120-024-01555-w
Real-world effectiveness and safety of trastuzumab-deruxtecan in Japanese patients with HER2-positive advanced gastric cancer (EN-DEAVOR study)
Erratum in
-
Correction: Real-world effectiveness and safety of trastuzumab-deruxtecan in Japanese patients with HER2-positive advanced gastric cancer (EN-DEAVOR study).Gastric Cancer. 2025 Jan;28(1):62. doi: 10.1007/s10120-024-01570-x. Gastric Cancer. 2025. PMID: 39607631 Free PMC article. No abstract available.
Abstract
Background: Trastuzumab-deruxtecan (T-DXd) was approved for the treatment of HER2-positive patients with advanced gastric cancer in Japan based on the results of the DESTINY-Gastric01 trial. This study aimed to collect real-world data and evaluate the effectiveness and safety of T-DXd.
Methods: Patients aged ≥ 20 years at the start of T-DXd administration with a histopathologically confirmed diagnosis of HER2-positive unresectable advanced or recurrent gastric or gastroesophageal junction (GEJ) adenocarcinoma that had worsened after chemotherapy were enrolled in this retrospective cohort study. Key outcomes included T-DXd treatment status, overall survival (OS), real-world progression-free survival (rwPFS), time to treatment failure (TTF), objective response rate and frequency of grade ≥ 3 adverse events (AEs).
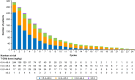
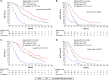
Results: Of the 312 patients included in the analysis, 75.3% were male, the median (range) age was 70.0 (27.0-89.0) years, 12.2% had an ECOG PS ≥ 2, 43.3% had ascites and the initial T-DXd dose was > 5.4- ≤ 6.4 mg/kg in 78.2% of patients. The median (95% confidence interval) OS, rwPFS and TTF (months) was 8.9 (8.0-11.0), 4.6 (4.0-5.1) and 3.9 (3.4-4.2), respectively. The response rate was 42.9% in patients with a target lesion. In total, 48.4% of patients experienced a grade ≥ 3 AE, 2.6% experienced grade 5 AEs and 60.9% experienced AEs leading to T-DXd dose adjustments (reduction: 36.9%, interruption: 34.0% or discontinuation: 23.7%). No new safety signals were detected.
Conclusions: T-DXd was effective and had a manageable safety profile as a third- or later-line treatment for patients with HER2-positive gastric or GEJ cancer in Japanese clinical practice.
Clinical trial registration: UMIN000049032.
Keywords: Effectiveness; Gastric cancer; HER2-positive; Japan; Post-marketing surveillance; Real world; Safety; Third- or later-line; Trastuzumab-deruxtecan (T-DXd).
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Hisato Kawakami has been a consultant for Astellas Pharma Inc., and Daiichi Sankyo; received honoraria from Bristol-Myers Squibb Co. Ltd., Eli Lilly Japan K.K., Ono Pharmaceutical Co. Ltd, Daiichi Sankyo Co. Ltd., Takeda Pharmaceutical Co. Ltd., Teijin Pharma Ltd, Otsuka Pharmaceutical Co., Ltd., GlaxoSmithKline K.K., Bayer Yakuhin Ltd, MSD K.K., Chugai Pharmaceutical Co. Ltd., Merck Biopharma Co., Ltd., Yakult Pharmaceutical Industry, Taiho Pharmaceutical Co. Ltd. and Nippon Kayaku Co. Ltd; and received research funding from Bristol-Myers Squibb Co. Ltd., Taiho Pharmaceutical Co. Ltd, Daiichi Sankyo, Eisai and Kobayashi Pharmaceuticals. Koki Nakanishi has received honoraria from Taiho Pharmaceutical, Ono Pharmaceutical, Daiichi Sankyo and Bristol-Myers Squibb. Akitaka Makiyama has been a consultant for Lilly Japan and received honoraria from Lilly Japan, Chugai Pharma, Takeda, Daiichi Sankyo, Taiho Pharmaceutical, Ono Pharmaceutical and Bristol-Myers Squibb Japan. Satoshi Morita reports honoraria from AstraZeneca Japan, Bristol-Myers Squibb, Chugai Pharma, Lilly Japan, Merck Sharp & Dohme, Nippon Boehringer Ingelheim, Ono Pharmaceutical, Pfizer and Eisai, research funding from Eisai and is one of the statistical editors for Gastric Cancer. Yukiya Narita has been a consultant for Daiichi Sankyo/AstraZeneca; been on the speakers’ bureau of Ono Pharmaceutical, Lilly, Taiho Pharmaceutical, Bristol-Myers Squibb and Daiichi Sankyo/AstraZeneca; and research funding from Ono Pharmaceutical, Daiichi Sankyo/AstraZeneca and Astellas Pharma. Naotoshi Sugimoto reports research funding from MSD, Ono Pharmaceutical, Taiho Pharmaceutical, Lilly Japan, Daiichi Sankyo, Sumitomo Dainippon Pharma, Chugai Pharma, BeiGene, Solasia Pharma, Astellas Pharma and Eisai. Keiko Minashi reports research funding from Ono Pharmaceutical, MSD, Astellas Pharma, BeiGene, Amgen and Daiichi Sankyo/UCB Japan. Yasuhiro Kodera has been a consultant for Daiichi Sankyo; received honoraria from Chugai Pharma, Yakult Honsha, Taiho Pharmaceutical, Ono Pharmaceutical, MSD K.K, Tsumura & Co., Daiichi Sankyo, Nippon Kayaku, Lilly Japan, Miyarisan Pharmaceutical, Olympus, AbbVie, Amgen, Bristol-Myers Squibb, Edwards Lifesciences and Covidien; research funding from Chugai Pharma, Daiichi Sankyo, Taiho Pharmaceutical, Abbott Japan, Lilly Japan, Kaken Pharmaceutical, Tsumura & Co., Covidien, Nippon Kayaku, Shionogi, Johnson & Johnson, Bayer, TOA Pharmaceuticals, AbbVie, EA Pharma, Pfizer and Otsuka and is the Editor-in-Chief of Gastric Cancer. Hiroki Kume, Keita Yamaguchi and Wataru Hashimoto are employees of Daiichi Sankyo Co. Ltd., Japan. Kei Muro has been a consultant or served as an adviser for Chugai Pharma, AstraZeneca, Ono Pharmaceutical, Amgen, Solasia Pharma; has received honoraria from Takeda, Chugai Pharma, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Bayer, Sanofi, Bristol-Myers Squibb, Daiichi Sankyo/UCB Japan, Ono Pharmaceutical, MSD, Daiichi Sankyo, Pfizer, Sanofi, Taiho Pharmaceutical, Astellas Pharma, Eisai, Chugai Pharma, Amgen, Novartis and Merck KGaA. Hirotaka Konishi, Motohiro Imano and Rin Inamoto have nothing to disclose. Ethics approval: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. The study protocol was approved by Nagoya University Bioethics Review Board (approval no. 2022–0170). Consent to participate: Informed consent was obtained from all patients; however, opt-out registration was permitted for patients from whom it was difficult to obtain written informed consent for a variety of reasons.
Figures



References
-
- International Agency for Research on Cancer (2024) World Health Organization. Cancer Today. Available at: https://gco.iarc.fr/today/data/factsheets/populations/392-japan-fact-she.... [Accessed on27th February 2024].
-
- Cancer statistics (2024) Accessed August 7, 2024. Available from https://ganjoho.jp/reg_stat/statistics/stat/cancer/5_stomach.html#anchor1
-
- Van Cutsem E, Bang YJ, Feng-Yi F, Xu JM, Lee KW, Jiao SC, Chong JL, López-Sanchez RI, Price T, Gladkov O, Stoss O, Hill J, Ng V, Lehle M, Thomas M, Kiermaier A, Rüschoff J. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer. 2015;18:476–84. 10.1007/s10120-014-0402-y. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

