High-Grade Progression, Sarcomatous Transformation, and/or Metastasis of Pituitary Neuroendocrine Neoplasms (PitNENs): The UCSF Experience
- PMID: 39388031
- PMCID: PMC11659330
- DOI: 10.1007/s12022-024-09829-w
High-Grade Progression, Sarcomatous Transformation, and/or Metastasis of Pituitary Neuroendocrine Neoplasms (PitNENs): The UCSF Experience
Abstract
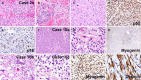
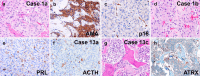
Pituitary neuroendocrine tumors (PitNET) that metastasize comprise ~ 0.2% of adenohypophyseal tumors are aggressive and are challenging to treat. However, many non-metastatic tumors are also aggressive. Herein, we review 21 specimens from 13 patients at UCSF with metastatic PitNETs (CSF or systemic, N = 7 patients), high-grade pituitary neuroendocrine neoplasms (HG-PitNEN, N = 4 patients), and/or PitNETs with sarcomatous transformation (PitNET-ST, N = 5 patients). We subtyped cases using the World Health Organization (WHO) and International Agency for Research on Cancer (IARC) criteria for neuroendocrine neoplasms (NENs). Lineage subtypes included acidophil stem cell, null cell, thyrotroph, corticotroph, lactotroph, and gonadotroph tumors. The median Ki-67 labeling index was 25% (range 5-70%). Lack of p16 was seen in 3 cases, with overexpression in 2. Strong diffuse p53 immunopositivity was present in 3 specimens from 2 patients. Loss of Rb expression was seen in 2 cases, with ATRX loss in one. Molecular analysis in 4 tumors variably revealed TERT alterations, homozygous CDKN2A deletion, aneuploidy, and mutations in PTEN, TP53, PDGFRB, and/or PIK3CA. Eight patients (62%) died of disease, 4 were alive at the last follow-up, and 1 was lost to the follow-up. All primary tumors had worrisome features, including aggressive lineage subtype, high mitotic count, and/or high Ki-67 indices. Additional evidence of high-grade progression included immunohistochemical loss of neuroendocrine, transcription factor, and/or hormone markers. We conclude that metastatic PitNET is not the only high-grade form of pituitary NEN. If further confirmed, these histopathologic and/or molecular features could provide advanced warning of biological aggressiveness and be applied towards a future grading scheme.
Keywords: Grading; Metastasis; Neuroendocrine neoplasms; Pituitary carcinoma; Pituitary neuroendocrine carcinoma; Pituitary neuroendocrine tumor; Pituitary sarcoma.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing Interests: The authors declare no competing interests.
Figures


References
-
- Ezzat, S., Asa, S. L., Couldwell, W. T., Barr, C. E., Dodge, W. E., Vance, M. L., & McCutcheon, I. E. (2004). The prevalence of pituitary adenomas: a systematic review. Cancer, 101(3), 613–619. https://doi-org.ucsf.idm.oclc.org/10.1002/cncr.20412 - DOI - PubMed
-
- Ostrom, Q. T., Gittleman, H., Truitt, G., Boscia, A., Kruchko, C., & Barnholtz-Sloan, J. S. (2018). CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011-2015. Neuro-oncology, 20(suppl_4), iv1–iv86. https://doi-org.ucsf.idm.oclc.org/10.1093/neuonc/noy131 - DOI - PMC - PubMed
-
- Raverot, G., Burman, P., McCormack, A., Heaney, A., Petersenn, S., Popovic, V., Trouillas, J., Dekkers, O. M., & European Society of Endocrinology (2018). European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. European journal of endocrinology, 178(1), G1–G24. https://doi-org.ucsf.idm.oclc.org/10.1530/EJE-17-0796 - DOI - PubMed
-
- Trouillas, J., Jaffrain-Rea, M. L., Vasiljevic, A., Dekkers, O., Popovic, V., Wierinckx, A., McCormack, A., Petersenn, S., Burman, P., Raverot, G., & Villa, C. (2020). Are aggressive pituitary tumors and carcinomas two sides of the same coin? Pathologists reply to clinician's questions. Reviews in endocrine & metabolic disorders, 21(2), 243–251. https://doi-org.ucsf.idm.oclc.org/10.1007/s11154-020-09562-9 - DOI - PubMed
-
- Kontogeorgos, G., Thodou, E., Osamura, R. Y., & Lloyd, R. V. (2022). High-risk pituitary adenomas and strategies for predicting response to treatment. Hormones (Athens, Greece), 21(1), 1–14. https://doi-org.ucsf.idm.oclc.org/10.1007/s42000-021-00333-y - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

