Sterile Intraocular Inflammation Associated With Faricimab
- PMID: 39388167
- PMCID: PMC11581583
- DOI: 10.1001/jamaophthalmol.2024.3828
Sterile Intraocular Inflammation Associated With Faricimab
Erratum in
-
Error in Text and Table.JAMA Ophthalmol. 2025 Jan 1;143(1):85. doi: 10.1001/jamaophthalmol.2024.5503. JAMA Ophthalmol. 2025. PMID: 39636618 Free PMC article. No abstract available.
Abstract
Importance: Randomized clinical trials are conducted to establish both drug safety and efficacy. However, evidence of adverse events associated with these drugs in the clinical practice setting can be of value at generating hypotheses regarding less common safety issues, even if causality cannot be determined.
Objective: To present and analyze cases of intraocular inflammation associated with faricimab therapy in patients referred to a single European institution.
Design, setting, and participants: This was a review starting in April of 2024 of an observational case series. Patients were from a single academic-based tertiary referral center in Switzerland. Included in the analysis were patients referred for intraocular inflammation soon after receiving a faricimab intravitreal injection between June 1, 2022, and March 5, 2024.
Exposure: Faricimab, 6 mg (0.05 mL of a 120-mg/mL solution), administrated for neovascular age-related macular degeneration or diabetic macular edema.
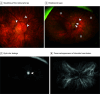
Main outcomes and measures: The systemic and ocular histories and imaging data available were reviewed. The following were evaluated: visual acuity measured with habitual correction using the Early Treatment of Diabetic Retinopathy Study charts before and after the event; intraocular pressure; patient symptoms; anterior, intermediate, or posterior location of the intraocular inflammation; and the presence of retinal vasculitis. Multimodal imaging including color fundus photographs, fluorescein angiograms, indocyanine green angiograms, and optical coherence tomography were reviewed.
Results: A total of 12 eyes from 7 patients (mean [SD] age, 73.3 [16.7] years; 4 female [57.1%]) over 22 months were identified as having noninfectious intraocular inflammation after intravitreal faricimab injections. Among these cases, in 2 eyes, retinal vasculitis was present together with anterior and posterior inflammation. One of the 2 eyes had an occlusive form of vasculitis of the arteries and veins, leading to subsequent macular capillary nonperfusion and clinically relevant irreversible vision deterioration from 20/80 to 20/2000. The remaining eyes were characterized by moderate anterior segment inflammation without substantial vision changes. The intraocular inflammation event occurred after a median (IQR) of 3.5 (2.0-4.3) faricimab injections. The median (IQR) interval between the last faricimab injection and the diagnosis of inflammation was 28 (24-38) days. Increased intraocular pressure of 30 mm Hg or higher was found in 3 eyes.
Conclusions and relevance: This case series highlights the occurrence of rare, but potentially severe, intraocular inflammation associated with faricimab therapy. Although these findings do not prove causality and can only generate hypotheses for future investigations, these results suggest the importance of continuous surveillance and monitoring for patients undergoing faricimab therapy to promptly identify and manage potential adverse events.
Conflict of interest statement
Figures



References
-
- Heier JS, Khanani AM, Quezada Ruiz C, et al. ; TENAYA and LUCERNE Investigators . Efficacy, durability, and safety of intravitreal faricimab up to every 16 weeks for neovascular age-related macular degeneration (TENAYA and LUCERNE): 2 randomized, double-masked, phase 3, noninferiority trials. Lancet. 2022;399(10326):729-740. doi:10.1016/S0140-6736(22)00010-1 - DOI - PubMed
-
- Wykoff CC, Abreu F, Adamis AP, et al. ; YOSEMITE and RHINE Investigators . Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): 2 randomized, double-masked, phase 3 trials. Lancet. 2022;399(10326):741-755. doi:10.1016/S0140-6736(22)00018-6 - DOI - PubMed

