Preventive Medications in Pediatric Migraine: A Network Meta-Analysis
- PMID: 39388181
- PMCID: PMC11581497
- DOI: 10.1001/jamanetworkopen.2024.38666
Preventive Medications in Pediatric Migraine: A Network Meta-Analysis
Abstract
Importance: Pediatric migraine substantially impacts quality of life and academic performance among children and adolescents. Understanding the efficacy and safety of pharmacological interventions for migraine prophylaxis in this population is crucial for developing effective treatment strategies.
Objective: To conduct a comprehensive network meta-analysis to evaluate the efficacy and safety associated with pharmacological treatments for pediatric migraine prophylaxis among pediatric patients with a migraine diagnosis and assess interventions involving various oral pharmacological interventions compared with each other and placebo.
Data sources: PubMed, Embase, and SCOPUS were searched for publications up to September 2023. Search terms and indexing were chosen to encompass relevant studies, focusing on randomized clinical trials in pediatric migraine prophylaxis.
Study selection: Inclusion criteria targeted randomized clinical trials involving pediatric patients with migraine. Studies were selected based on their examination of oral pharmacological interventions. The search yielded an initial 9162 citations.
Data extraction and synthesis: Data extraction adhered to Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines. Five investigators independently extracted study data into a spreadsheet in duplicate. Study-level estimates were calculated, employing a random-effects model for primary and secondary outcomes due to identified heterogeneity. Data analysis was conducted from December 2023 to March 2024.
Main outcomes and measures: The primary outcome was migraine frequency (number of attacks per month). Secondary outcomes included a 50% or greater responder rate, headache duration, headache intensity, and disability (assessed by pediatrics migraine-specific disability tool). Adverse events were also evaluated.
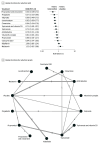
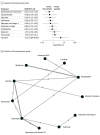
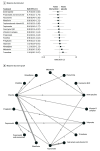
Results: The analysis incorporated 45 trials with 3771 participants. Compared with placebo, pregabalin (ratio of means [RoM], 0.38; 95% CI, 0.18-0.79) and topiramate with vitamin D3 (RoM, 0.44; 95% CI, 0.30-0.65) were associated with reduction in migraine frequency. Flunarizine (RoM, 0.46; 95% CI, 0.26-0.81), levetiracetam (RoM, 0.47; 95% CI, 0.30-0.72), riboflavin (RoM, 0.50; 95% CI, 0.32-0.77), cinnarizine (RoM, 0.64; 95% CI, 0.46-0.88), topiramate (RoM, 0.70; 95% CI, 0.55-0.89), and amitriptyline (RoM, 0.73; 95% CI, 0.54-0.97) were also associated with reduction in migraine frequency, but these findings were drawn from individual studies. For the 50% or greater responder rate, flunarizine and α-lipoic acid (risk ratio [RR], 8.73; 95% CI, 2.44-31.20), flunarizine (RR, 4.00; 95% CI, 1.38-11.55), pregabalin (RR, 1.88; 95% CI, 1.13-3.14), and cinnarizine (RR, 1.46; 95% CI, 1.04-2.05) were associated with significantly greater effectiveness than placebo. Compared with placebo, propranolol and cinnarizine (RoM, 0.45; 95% CI, 0.28-0.72), pregabalin (RoM, 0.57; 95% CI, 0.33-0.96), valproate (RoM, 0.60; 95% CI, 0.49-0.72), levetiracetam (RoM, 0.62; 95% CI, 0.50-0.77), and cinnarizine (RoM, 0.64; 95% CI, 0.54-0.76) were significantly associated with reduction in headache intensity. However, no treatments were associated with significant improvements in quality of life or reduction of the duration of migraine attacks. Adverse events were higher with amitriptyline (RR, 3.81; 95% CI, 1.41-10.32), topiramate (RR, 4.34; 95% CI, 1.60-11.75), and valproate (RR, 5.93; 95% CI, 1.93-18.23) compared with placebo.
Conclusions and relevance: In this network meta-analysis of randomized clinical trials, topiramate and pregabalin were associated with reduction in headache frequency and intensity. Although there were also other drugs that showed statistically significant results (flunarizine, riboflavin, amitriptyline, and cinnarizine), more studies were required for a robust conclusion. None of the drugs were associated with improved quality of life or attack duration, underscoring the need for further research to develop more comprehensive treatment strategies and explore the potential of combination therapies, especially those involving vitamins. Future studies should focus on validating these findings and expanding the treatment landscape for pediatric migraine management.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

