O-GlcNAcylation of ATP-citrate lyase couples glucose supply to lipogenesis for rapid tumor cell proliferation
- PMID: 39388261
- PMCID: PMC11494317
- DOI: 10.1073/pnas.2402674121
O-GlcNAcylation of ATP-citrate lyase couples glucose supply to lipogenesis for rapid tumor cell proliferation
Abstract
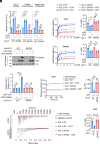
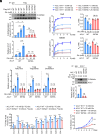
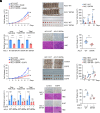
Elevated lipid synthesis is one of the best-characterized metabolic alterations in cancer and crucial for membrane expansion. As a key rate-limiting enzyme in de novo fatty acid synthesis, ATP-citrate lyase (ACLY) is frequently up-regulated in tumors and regulated by posttranslational modifications (PTMs). Despite emerging evidence showing O-GlcNAcylation on ACLY, its biological function still remains unknown. Here, we observed a significant upregulation of ACLY O-GlcNAcylation in various types of human tumor cells and tissues and identified S979 as a major O-GlcNAcylation site. Importantly, S979 O-GlcNAcylation is required for substrate CoA binding and crucial for ACLY enzymatic activity. Moreover, it is sensitive to glucose fluctuation and decisive for fatty acid synthesis as well as tumor cell proliferation. In response to EGF stimulation, both S979 O-GlcNAcylation and previously characterized S455 phosphorylation played indispensable role in the regulation of ACLY activity and cell proliferation; however, they functioned independently from each other. In vivo, streptozocin treatment- and EGFR overexpression-induced growth of xenograft tumors was mitigated once S979 was mutated. Collectively, this work helps comprehend how cells interrogate the nutrient enrichment for proliferation and suggests that although mammalian cell proliferation is controlled by mitogen signaling, the ancient nutrition-sensing mechanism is conserved and still efficacious in the cells of multicellular organisms.
Keywords: ATP-citrate lyase; CoA association; O-GlcNAcylation; fatty acid synthesis; tumor cell proliferation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
MeSH terms
Substances
Grants and funding
- 32101028/MOST | National Natural Science Foundation of China (NSFC)
- 32070896/MOST | National Natural Science Foundation of China (NSFC)
- 2412021QD013/MOE | Fundamental Research Funds for the Central Universities (Fundamental Research Fund for the Central Universities)
- QT202105/The Young Scientific and Technological Talents Support Project of Jilin Province
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

