Natural variation in age-related dopamine neuron degeneration is glutathione dependent and linked to life span
- PMID: 39388265
- PMCID: PMC11494315
- DOI: 10.1073/pnas.2403450121
Natural variation in age-related dopamine neuron degeneration is glutathione dependent and linked to life span
Abstract
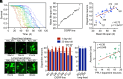
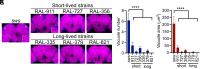
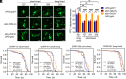
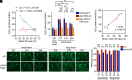
Aging is the biggest risk factor for Parkinson's disease (PD), suggesting that age-related changes in the brain promote dopamine neuron vulnerability. It is unclear, however, whether aging alone is sufficient to cause significant dopamine neuron loss, and if so, how this intersects with PD-related neurodegeneration. Here, through examining a large collection of naturally varying Drosophila strains, we find a strong relationship between life span and age-related dopamine neuron loss. Strains with naturally short-lived animals exhibit a loss of dopamine neurons without generalized neurodegeneration, while animals from long-lived strains retain dopamine neurons across age. Metabolomic profiling reveals lower glutathione levels in short-lived strains which is associated with elevated levels of reactive oxygen species (ROS), sensitivity to oxidative stress, and vulnerability to silencing the familial PD gene parkin. Strikingly, boosting neuronal glutathione levels via glutamate-cysteine ligase (Gcl) overexpression is sufficient to normalize ROS levels, extend life span, and block dopamine neurons loss in short-lived backgrounds, demonstrating that glutathione deficiencies are central to neurodegenerative phenotypes associated with short longevity. These findings may be relevant to human PD pathogenesis, where glutathione depletion is reported to occur in the idiopathic PD patient brain through unknown mechanisms. Building on this, we find reduced expression of the Gcl catalytic subunit in both Drosophila strains vulnerable to age-related dopamine neuron loss and in the human brain from familial PD patients harboring the common LRRK2 G2019S mutation. Our study across Drosophila and human PD systems suggests that glutathione synthesis and levels play a conserved role in regulating age-related dopamine neuron health.
Keywords: Parkinson’s disease; aging; glutathione; neurodegeneration.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Update of
-
Natural Variation in Age-Related Dopamine Neuron Degeneration is Glutathione-Dependent and Linked to Life Span.bioRxiv [Preprint]. 2024 Feb 14:2024.02.12.580013. doi: 10.1101/2024.02.12.580013. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2403450121. doi: 10.1073/pnas.2403450121. PMID: 38405950 Free PMC article. Updated. Preprint.
Similar articles
-
Natural Variation in Age-Related Dopamine Neuron Degeneration is Glutathione-Dependent and Linked to Life Span.bioRxiv [Preprint]. 2024 Feb 14:2024.02.12.580013. doi: 10.1101/2024.02.12.580013. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2403450121. doi: 10.1073/pnas.2403450121. PMID: 38405950 Free PMC article. Updated. Preprint.
-
PARIS induced defects in mitochondrial biogenesis drive dopamine neuron loss under conditions of parkin or PINK1 deficiency.Mol Neurodegener. 2020 Mar 5;15(1):17. doi: 10.1186/s13024-020-00363-x. Mol Neurodegener. 2020. PMID: 32138754 Free PMC article.
-
Dietary Amino Acids Impact LRRK2-Induced Neurodegeneration in Parkinson's Disease Models.J Neurosci. 2020 Aug 5;40(32):6234-6249. doi: 10.1523/JNEUROSCI.2809-19.2020. Epub 2020 Jun 30. J Neurosci. 2020. PMID: 32605938 Free PMC article.
-
Parkin loss-of-function pathology: Premature neuronal senescence induced by high levels of reactive oxygen species?Mech Ageing Dev. 2017 Jan;161(Pt A):112-120. doi: 10.1016/j.mad.2016.06.008. Epub 2016 Jun 29. Mech Ageing Dev. 2017. PMID: 27374431 Review.
-
The role of oxidative stress in Parkinson's disease.J Parkinsons Dis. 2013;3(4):461-91. doi: 10.3233/JPD-130230. J Parkinsons Dis. 2013. PMID: 24252804 Free PMC article. Review.
Cited by
-
Aging disrupts the coordination between mRNA and protein expression in mouse and human midbrain.bioRxiv [Preprint]. 2024 Jun 1:2024.06.01.596950. doi: 10.1101/2024.06.01.596950. bioRxiv. 2024. Update in: Mol Psychiatry. 2025 Jul;30(7):3039-3054. doi: 10.1038/s41380-025-02909-1. PMID: 38854057 Free PMC article. Updated. Preprint.
-
LRRK2-mediated mitochondrial dysfunction in Parkinson's disease.Biochem J. 2025 May 28;482(11):721-39. doi: 10.1042/BCJ20253062. Biochem J. 2025. PMID: 40440058 Free PMC article. Review.
-
Ferroptosis and Iron Homeostasis: Molecular Mechanisms and Neurodegenerative Disease Implications.Antioxidants (Basel). 2025 Apr 28;14(5):527. doi: 10.3390/antiox14050527. Antioxidants (Basel). 2025. PMID: 40427409 Free PMC article. Review.
-
Aging-Associated Amyloid-β Plaques and Neuroinflammation in Bottlenose Dolphins (Tursiops truncatus) and Novel Cognitive Health-Supporting Roles of Pentadecanoic Acid (C15:0).Int J Mol Sci. 2025 Apr 16;26(8):3746. doi: 10.3390/ijms26083746. Int J Mol Sci. 2025. PMID: 40332352 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- S10 OD021562/OD/NIH HHS/United States
- R56 AG049494/AG/NIA NIH HHS/United States
- R01 AG063371/AG/NIA NIH HHS/United States
- S10 OD021562/CD/ODCDC CDC HHS/United States
- T32 AG055378/AG/NIA NIH HHS/United States
- T32AG055378/HHS | National Institutes of Health (NIH)
- R01AG063371/HHS | National Institutes of Health (NIH)
- R01 NS119226/NS/NINDS NIH HHS/United States
- Foundation funds/Oregon Health and Science University (OHSU)
- P30AG013280/HHS | National Institutes of Health (NIH)
- P30 NS061800/NS/NINDS NIH HHS/United States
- P30 AG013280/AG/NIA NIH HHS/United States
- R01 AG049494/AG/NIA NIH HHS/United States
- R56AG049494/HHS | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

