A receptor for dual ligands governs plant immunity and hormone response and is targeted by a nematode effector
- PMID: 39388275
- PMCID: PMC11494329
- DOI: 10.1073/pnas.2412016121
A receptor for dual ligands governs plant immunity and hormone response and is targeted by a nematode effector
Abstract
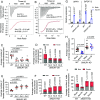
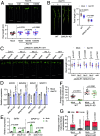
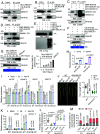
In this study, we show that the potato (Solanum tuberosum) pattern recognition receptor (PRR) NEMATODE-INDUCED LEUCINE-RICH REPEAT (LRR)-RLK1 (StNILR1) functions as a dual receptor, recognizing both nematode-associated molecular pattern ascaroside #18 (Ascr18) and plant hormone brassinosteroid (BR) to activate two different physiological outputs: pattern-triggered immunity (PTI) and BR response. Ascr18/BR-StNILR1 signaling requires the coreceptor potato BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED RECEPTOR KINASE 1 (StBAK1) and perception of either ligand strengthens StNILR1 interaction with StBAK1 in plant cells. Significantly, the parasitically successful potato cyst nematode (Globodera pallida) utilizes the effector RHA1B, which is a functional ubiquitin ligase, to target StNILR1 for ubiquitination-mediated proteasome-dependent degradation, thereby countering Ascr18/BR-StNILR1-mediated PTI in potato and facilitating nematode parasitism. These findings broaden our understanding of PRR specificity and reveal a nematode parasitic mechanism that targets a PTI signaling pathway.
Keywords: Ascr18; BR; RHA1B; StNILR1; dual receptor.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
-
- Zhou J.-M., Zhang Y., Plant immunity: Danger perception and signaling. Cell 181, 978–989 (2020). - PubMed
-
- Tanaka K., Heil M., Damage-associated molecular patterns (DAMPs) in plant innate immunity: Applying the danger model and evolutionary perspectives. Annu. Rev. Phytopathol. 59, 53–75 (2021). - PubMed
-
- Zhang C., Xie Y., He P., Shan L., Unlocking nature’s defense: Plant pattern recognition receptors as guardians against pathogenic threats. Mol. Plant Microbe Interact. 37, 73–83 (2024). - PubMed
MeSH terms
Substances
Grants and funding
- 2022-67014-36138/USDA | National Institute of Food and Agriculture (NIFA)
- 2022-67014-36138/USDA | National Institute of Food and Agriculture (NIFA)
- 2022-67014-36138/USDA | National Institute of Food and Agriculture (NIFA)
- AP19PPQFO000495/USDA | Animal and Plant Health Inspection Service (APHIS)
- AP19PPQFO000495/USDA | Animal and Plant Health Inspection Service (APHIS)
- AP19PPQFO000495/USDA | Animal and Plant Health Inspection Service (APHIS)
- AP19PPQFO000495/USDA | Animal and Plant Health Inspection Service (APHIS)
- 2022-51181-38450/USDA | National Institute of Food and Agriculture (NIFA)
- 2022-51181-38450/USDA | National Institute of Food and Agriculture (NIFA)
- 2022-51181-38450/USDA | National Institute of Food and Agriculture (NIFA)
- 2022-51181-38450/USDA | National Institute of Food and Agriculture (NIFA)
- 2024-67013-42425/USDA | National Institute of Food and Agriculture (NIFA)
LinkOut - more resources
Full Text Sources

