Humoral and T-cell-mediated responses to an insect-specific flavivirus-based Zika virus vaccine candidate
- PMID: 39388457
- PMCID: PMC11495591
- DOI: 10.1371/journal.ppat.1012566
Humoral and T-cell-mediated responses to an insect-specific flavivirus-based Zika virus vaccine candidate
Abstract
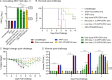
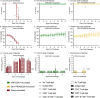
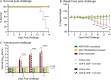
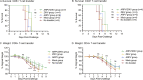
Flaviviruses represent a significant global health threat and relatively few licensed vaccines exist to protect against them. Insect-specific flaviviruses (ISFVs) are incapable of replication in humans and have emerged as a novel and promising tool for flavivirus vaccine development. ISFV-based flavivirus vaccines have shown exceptional safety, immunogenicity, and efficacy, however, a detailed assessment of the correlates of protection and immune responses induced by these vaccines are still needed for vaccine optimization. Here, we explore the mechanisms of protective immunity induced by a previously created pre-clinical Zika virus (ZIKV) vaccine candidate, called Aripo/Zika (ARPV/ZIKV). In brief, immunocompromised IFN-αβR-/- mice passively immunized with ARPV/ZIKV immune sera experienced protection after lethal ZIKV challenge, although this protection was incomplete. ARPV/ZIKV-vaccinated IFN-αβR-/- mice depleted of CD4+ or CD8+ T-cells at the time of ZIKV challenge showed no morbidity or mortality. However, the adoptive transfer of ARPV/ZIKV-primed T-cells into recipient IFN-αβR-/- mice resulted in a two-day median increase in survival time compared to controls. Altogether, these results suggest that ARPV/ZIKV-induced protection is primarily mediated by neutralizing antibodies at the time of challenge and that T-cells may play a comparatively minor but cumulative role in the protection observed. Lastly, ARPV/ZIKV-vaccinated Tcra KO mice, which are deficient in T-cell responses, experienced significant mortality post-challenge. These results suggest that ARPV/ZIKV-induced cell-mediated responses are critical for development of protective immune responses at vaccination. Despite the strong focus on neutralizing antibody responses to novel flavivirus vaccine candidates, these results suggest that cell-mediated responses induced by ISFV-based vaccines remain important to overall protective responses.
Copyright: © 2024 Porier et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Update of
-
Humoral and T-cell-mediated responses to a pre-clinical Zika vaccine candidate that utilizes a unique insect-specific flavivirus platform.bioRxiv [Preprint]. 2023 Mar 1:2023.03.01.530296. doi: 10.1101/2023.03.01.530296. bioRxiv. 2023. Update in: PLoS Pathog. 2024 Oct 10;20(10):e1012566. doi: 10.1371/journal.ppat.1012566. PMID: 36909623 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

