CRISPR/Cas9 gene editing in induced pluripotent stem cells to investigate the feline hypertrophic cardiomyopathy causing MYBPC3/R820W mutation
- PMID: 39388496
- PMCID: PMC11466433
- DOI: 10.1371/journal.pone.0311761
CRISPR/Cas9 gene editing in induced pluripotent stem cells to investigate the feline hypertrophic cardiomyopathy causing MYBPC3/R820W mutation
Abstract
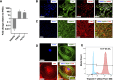
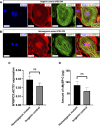
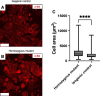
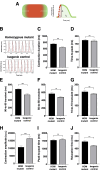
Hypertrophic cardiomyopathy (HCM) is the most common heart disease in domestic cats, often leading to congestive heart failure and death, with current treatment strategies unable to reverse or prevent progression of the disease. The underlying pathological processes driving HCM remain unclear, which hinders novel drug discovery. The aim of this study was to generate a cellular model of the feline HCM-causing MYBPC3 mutation R820W. Using CRISPR/Cas9 gene editing we introduced the R820W mutation into a human induced pluripotent stem cell (iPSC) line. We differentiated both homozygous mutant clones and isogenic control clones to cardiomyocytes (iPSC-CMs). Protein quantification indicated that haploinsufficiency is not the disease mechanism of the mutation. Homozygous mutant iPSC-CMs had a larger cell area than isogenic controls, with the sarcomere structure and incorporation of cMyBP-C appearing similar between mutant and control iPSC-CMs. Contraction kinetic analysis indicated that homozygous iPSC-CMs have impaired relaxation and are hypocontractile compared to isogenic control iPSC-CMs. In summary, we demonstrate successful generation of an iPSC model of a feline MYBPC3 mutation, with the cellular model recapitulating aspects of HCM including cellular hypertrophy and impaired relaxation kinetics. We anticipate that further study of this model will lead to improved understanding of the disease-causing molecular mechanism, ultimately leading to novel drug discovery.
Copyright: © 2024 Dutton et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Fox PR, Keene BW, Lamb K, Schober KA, Chetboul V, Luis Fuentes V, et al.. International collaborative study to assess cardiovascular risk and evaluate long‐term health in cats with preclinical hypertrophic cardiomyopathy and apparently healthy cats: The REVEAL Study. J Vet Intern Med. 2018;32: 930–943. doi: 10.1111/jvim.15122 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

