Short QT intervals in African lions
- PMID: 39388603
- PMCID: PMC11607619
- DOI: 10.1113/EP092203
Short QT intervals in African lions
Abstract
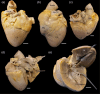
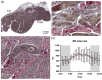
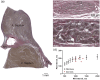
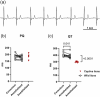
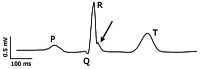
The cardiac conduction system in large carnivores, such as the African lion (Panthera leo), represents a significant knowledge gap in both veterinary science and in cardiac electrophysiology. Short QT intervals have been reported from zoo-kept, anaesthetized lions, and our goal was to record the first ECGs from wild, conscious lions roaming freely, and compare them to zoo-kept lions under the hypothesis that short QT is unique to zoo-kept lions. Macroscopic and histological examinations were performed on heart tissue removed from nine healthy zoo lions. ECGs were recorded from the nine anaesthetized zoo-kept lions, and from 15 anaesthetized and conscious wild lions in Africa. Our histological and topographical description of the lion's heart matched what has previously been published. In conscious lions, the ECG recordings revealed a mean heart rate of 70 ± 4 beats/min, with faster heart rates during the night. PQ and QT intervals were heart rate dependent in the conscious lions. Interestingly, QT intervals recorded in wild lions were markedly longer than QT intervals from zoo lions (398 ± 40 vs. 297 ± 9 ms, respectively; P < 0.0001). Anaesthesia or heart rate did not account for this difference. We provide a comprehensive description of the cardiac anatomy and electrophysiology of wild and zoo-kept lions. QT intervals were significantly shorter in zoo lions, suggesting functional disparities in cardiac electrophysiology between wild and zoo-kept lions, potentially related to physical fitness. These findings underscore the plasticity of cardiac electrophysiology and may be of value when reintroducing endangered species into the wild and when managing lions in human care.
Keywords: Panthera leo; Purkinje; QT; circadian; feline; predator.
© 2024 The Author(s). Experimental Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
None declared.
Figures

References
-
- Bazett, H. C. (1920). An analysis of the time‐relations of electrocardiograms. Heart, 7, 353–370.
-
- Bertelsen . (2018). Issues surrounding surplus animals in zoos. Fowler's zoo and wild animal medicine, current therapy. pp. 134–137. Elsevier.
-
- Calloe, K. , Cordeiro, J. M. , Di Diego, J. M. , Hansen, R. S. , Grunnet, M. , Olesen, S. P. , & Antzelevitch, C. (2008). A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovascular Research, 81(4), 686–694. - PMC - PubMed
-
- Christou, G. A. , Vlahos, A. P. , Christou, K. A. , Mantzoukas, S. , Drougias, C. A. , & Christodoulou, D. K. (2022). Prolonged QT interval in athletes: Distinguishing between pathology and physiology. Cardiology, 147(5‐6), 578–586. - PubMed
-
- Elbrønd, V. S. , Thomsen, M. B. , Isaksen, J. L. , Lunde, E. D. , Vincenti, S. , Wang, T. , Tranum‐Jensen, J. , & Calloe, K. (2023). Intramural Purkinje fibers facilitate rapid ventricular activation in the equine heart. Acta Physiologica, 237(3), 13925. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
