Real-World Immune-Related Adverse Events in Patients With Early Triple-Negative Breast Cancer Who Received Pembrolizumab
- PMID: 39388649
- PMCID: PMC12208142
- DOI: 10.1200/OP.24.00371
Real-World Immune-Related Adverse Events in Patients With Early Triple-Negative Breast Cancer Who Received Pembrolizumab
Abstract
Purpose: The addition of pembrolizumab to chemotherapy in high-risk early triple-negative breast cancer (TNBC) improves cancer outcomes. However, pembrolizumab induces varied immune-related adverse events (irAEs) where some can be severe or lifelong. This retrospective study describes real-world patterns of irAEs in patients with TNBC who received pembrolizumab.
Methods: We evaluated irAEs in patients with TNBC from a comprehensive cancer center and a community hospital who received pembrolizumab with chemotherapy between 2021 and 2023, excluding those enrolled in clinical trials. We used national guidelines to grade toxicities. Logistic regression assessed the effect of clinicopathologic variables on irAEs adjusting for covariates.
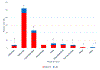
Results: We identified 233 patients with a median age of 51 years, 62% had stage II TNBC, 35% had stage III TNBC, 25% were Hispanic, 21% were Black, and 42% were White. Eighty patients (34%) developed 100 separate irAEs. The most common irAEs were endocrinopathies (52%) and GI (23%); there were 26 grade ≥3 irAEs, which all resulted in hospitalization, the most common being GI (13 instances); 45 required systemic steroids, 16 required additional immunosuppressive therapy, and 32 patients discontinued pembrolizumab because of irAEs. Two patients who developed colitis eventually died due to complications. Most (67 instances) irAEs were unresolved at the time of last follow-up, but 55% (37/67) had improved to grade 1. No clinicopathologic factors were associated with the development or severity of irAEs.
Conclusion: In this real-world diverse population, we observed rates of irAEs comparable with KEYNOTE-522, where endocrinopathies were the most prevalent, but GI irAEs were also prevalent and severe. This emphasizes a critical issue as pembrolizumab is increasingly being used in early TNBC and could have long-term survivorship implications.
Conflict of interest statement
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

