Tirzepatide as an innovative treatment strategy in a pre-clinical model of obesity-driven endometrial cancer
- PMID: 39388742
- PMCID: PMC12419173
- DOI: 10.1016/j.ygyno.2024.10.004
Tirzepatide as an innovative treatment strategy in a pre-clinical model of obesity-driven endometrial cancer
Abstract
Objective: Interventions that combat obesity and its associated metabolic perturbations may decrease incidence and improve outcomes of endometrial cancer (EC). Potential options for weight loss include pharmacotherapeutic interventions such as tirzepatide, a dual-acting glucagon-like peptide 1 (GLP-1) and gastric inhibitory polypeptide (GIP) receptor agonist. Given this, we explored the anti-obesity and anti-tumorigenic effects of tirzepatide in our pre-clinical mouse model of endometrioid EC.
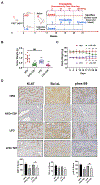
Methods: Starting at 4 weeks of age, Lkb1fl/flp53fl/fl mice were fed a low-fat diet vs a high-fat diet to generate a lean or obese phenotype. Nine weeks after induction of EC, obese and lean mice were randomized to receive tirzepatide for 4 weeks. Body and tumor weights, tumor transcriptomic and metabolomic profiles, and serum metabolic markers and chemokines were assessed.
Results: Both obese and lean mice began to lose body weight after 2 weeks of tirzepatide treatment, ultimately achieving a significant weight loss of 20.1 % in obese mice and 16.8 % in lean mice. Tirzepatide improved obesity-induced serum adiponectin, leptin, GIP, and C-reactive protein levels. Furthermore, tirzepatide relative to vehicle, effectively reduced tumor growth in obese and lean mice, inhibited the ErbB signaling and glycolysis/gluconeogenesis in tumors of obese mice, and increased O-linked glycosylation biosynthesis and phospholipase D signaling in tumors of lean mice.
Conclusion: Tirzepatide decreased both mouse weight and tumor growth via effects on metabolic and immune pathways in the EC tumors that differed between obese and lean mice. This novel weight loss treatment deserves further evaluation as an innovative strategy in the management of EC.
Keywords: Body weight; Endometrial cancer; Metabolon; Obesity; Tirzepatide.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors did not disclose potential conflicts of interest.
Figures



References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. - PubMed
-
- Agnew HJ, Kitson SJ, Crosbie EJ. Gynecological malignancies and obesity. Best Pract Res Clin Obstet Gynaecol. 2023;88:102337. - PubMed
-
- Kitson SJ, Evans DG, Crosbie EJ. Identifying High-Risk Women for Endometrial Cancer Prevention Strategies: Proposal of an Endometrial Cancer Risk Prediction Model. Cancer Prev Res (Phila). 2017;10(1):1–13. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

