SARS-CoV-2 antigen rapid detection tests: test performance during the COVID-19 pandemic and the impact of COVID-19 vaccination
- PMID: 39388783
- PMCID: PMC11663747
- DOI: 10.1016/j.ebiom.2024.105394
SARS-CoV-2 antigen rapid detection tests: test performance during the COVID-19 pandemic and the impact of COVID-19 vaccination
Abstract
Background: SARS-CoV-2 antigen rapid detection tests (RDTs) emerged as point-of-care diagnostics alongside reverse transcription polymerase chain reaction (RT-qPCR) as reference.
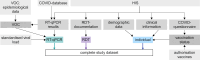
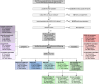
Methods: In a prospective performance assessment from 12 November 2020 to 30 June 2023 at a single centre tertiary care hospital, the sensitivity and specificity (primary endpoints) of RDTs from three manufacturers (NADAL®, Panbio™, MEDsan®) were compared to RT-qPCR as reference standard among patients, accompanying persons and staff aged ≥ six month in large-scale, clinical screening use. Regression models were used to assess influencing factors on RDT performance (secondary endpoints).
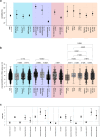
Findings: Among 78,798 paired RDT/RT-qPCR results analysed, overall RDT sensitivity was 34.5% (695/2016; 95% CI 32.4-36.6%), specificity 99.6% (76,503/76,782; 95% CI 99.6-99.7%). Over the pandemic course, sensitivity decreased in line with a lower rate of individuals showing typical COVID-19 symptoms. The lasso regression model showed that a higher viral load and typical COVID-19 symptoms were directly significantly correlated with the likelihood of a positive RDT result in SARS-CoV-2 infection, whereas age, sex, vaccination status, and the Omicron VOC were not.
Interpretation: The decline in RDT sensitivity throughout the pandemic can primarily be attributed to the reduced prevalence of symptomatic infections among vaccinated individuals and individuals infected with Omicron VOC. RDTs remain valuable for detecting SARS-CoV-2 in symptomatic individuals and offer potential for detecting other respiratory pathogens in the post-pandemic era, underscoring their importance in infection control efforts.
Funding: German Federal Ministry of Education and Research (BMBF), Free State of Bavaria, Bavarian State Ministry of Health and Care.
Keywords: COVID-19 symptoms; COVID-19 vaccination; SARS-CoV-2 rapid antigen detection test; Test performance; Viral load; Virus variants of concern.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Daniel Zeller receives honoraria from Angelini Pharma and Novartis outside of the submitted work. Manuel Krone receives honoraria from Abbott, GSK, Pfizer, and Sanofi outside of the submitted work. None of the other authors have any conflicts of interest to declare.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

