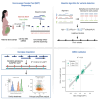
Utilizing non-invasive prenatal test sequencing data for human genetic investigation
- PMID: 39389018
- PMCID: PMC11602596
- DOI: 10.1016/j.xgen.2024.100669
Utilizing non-invasive prenatal test sequencing data for human genetic investigation
Abstract
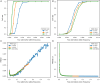
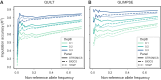
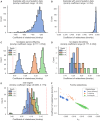
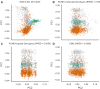
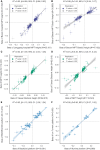
Non-invasive prenatal testing (NIPT) employs ultra-low-pass sequencing of maternal plasma cell-free DNA to detect fetal trisomy. Its global adoption has established NIPT as a large human genetic resource for exploring genetic variations and their associations with phenotypes. Here, we present methods for analyzing large-scale, low-depth NIPT data, including customized algorithms and software for genetic variant detection, genotype imputation, family relatedness, population structure inference, and genome-wide association analysis of maternal genomes. Our results demonstrate accurate allele frequency estimation and high genotype imputation accuracy (R2>0.84) for NIPT sequencing depths from 0.1× to 0.3×. We also achieve effective classification of duplicates and first-degree relatives, along with robust principal-component analysis. Additionally, we obtain an R2>0.81 for estimating genetic effect sizes across genotyping and sequencing platforms with adequate sample sizes. These methods offer a robust theoretical and practical foundation for utilizing NIPT data in medical genetic research.
Keywords: NIPT-human-genetics workflow; allele frequency estimation; cell-free DNA; family relatedness; genome-wide association analysis; genotype imputation; low-pass whole-genome sequencing; non-invasive prenatal test; population structure; variant detection.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures