When is the use of intravenous immunoglobulin appropriate in immune thrombocytopenia?
- PMID: 39389921
- PMCID: PMC11637736
- DOI: 10.1111/bjh.19817
When is the use of intravenous immunoglobulin appropriate in immune thrombocytopenia?
Abstract
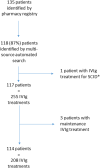
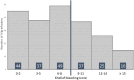
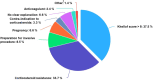
Intravenous immunoglobulin (IVIg) is the gold standard treatment for severe cases of immune thrombocytopenia (ITP). However, its cost, limited duration of efficacy and market supply tension have led French guidelines to reserve IVIg for ITP patients with formal contra-indications to corticosteroids, with French bleeding score ('Khellaf score') > 8, and corticosteroid-resistant patients either with Khellaf score ≤ 8 or in preparation for an invasive procedure or during pregnancy. We studied the prescribing practices of IVIg for ITP in real-life conditions and assessed their compliance with French guidelines. A monocentric retrospective study was conducted between 2016 and 2020 among 114 patients hospitalized in our unit, for a total of 208 IVIg treatments. In 37% of cases, the Khellaf score was >8, validating IVIg prescription according to French guidelines. In the remaining cases, reasons noted for use of IVIg included corticosteroid resistance (33.7%), preparation for an invasive procedure (8.5%), context of pregnancy (6.6%) and contra-indication to corticosteroids (3.3%). After analysis, IVIg prescription was considered valid according to current French guidelines in 84.4% of cases. Non-compliant IVIg prescription was more frequent in younger patients (p = 0.027). Concomitant anti-coagulation was also noted as an argument for IVIg prescription outside of the current French guidelines.
Keywords: immune thrombocytopenia; intravenous immunoglobulin.
© 2024 The Author(s). British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
No relevant conflicts of interest to declare.
Figures

References
-
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. - PubMed
-
- Godeau B, Chevret S, Varet B, Lefrère F, Zini JM, Bassompierre F, et al. Intravenous immunoglobulin or high‐dose methylprednisolone, with or without oral prednisone, for adults with untreated severe autoimmune thrombocytopenic purpura: a randomised, multicentre trial. Lancet. 2002;359(9300):23–29. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

