The SRC-family serves as a therapeutic target in triple negative breast cancer with acquired resistance to chemotherapy
- PMID: 39390250
- PMCID: PMC11554838
- DOI: 10.1038/s41416-024-02875-5
The SRC-family serves as a therapeutic target in triple negative breast cancer with acquired resistance to chemotherapy
Abstract
Background: Resistance to chemotherapy, combined with heterogeneity among resistant tumors, represents a significant challenge in the clinical management of triple negative breast cancer (TNBC). By dissecting molecular pathways associated with treatment resistance, we sought to define patient sub-groups and actionable targets for next-line treatment.
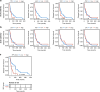
Methods: Bulk RNA sequencing and reverse phase protein array profiling were performed on isogenic patient-derived xenografts (PDX) representing paclitaxel-sensitive and -resistant tumors. Pathways identified as upregulated in the resistant model were further explored as targets in PDX explants. Their clinical relevance was assessed in two distinct patient cohorts (NeoAva and MET500).
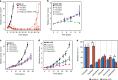
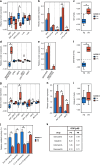
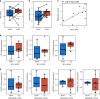
Results: Increased activity in signaling pathways involving SRC-family kinases (SFKs)- and MAPK/ERK was found in treatment resistant PDX, with targeted inhibitors being significantly more potent in resistant tumors. Up-regulation of SFKs- and MAPK/ERK-pathways was also detected in a sub-group of chemoresistant patients after neoadjuvant treatment. Furthermore, High SFK expression (of either SRC, FYN and/or YES1) was detected in metastatic lesions of TNBC patients with fast progressing disease (median disease-free interval 27 vs 105 months).
Conclusions: Upregulation of SFK-signaling is found in a subset of chemoresistant tumors and is persistent in metastatic lesions. Based on pre-clinical results, these patients may respond favorably to treatment targeting SFKs.
© 2024. The Author(s).
Conflict of interest statement
Figures


References
-
- Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. - PubMed
-
- Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N. Engl J Med. 2020;382:810–21. - PubMed
-
- Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kümmel S, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N. Engl J Med. 2022;386:556–67. - PubMed
MeSH terms
Substances
Grants and funding
- 275437/Norges Forskningsråd (Research Council of Norway)
- 847912/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 859962/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- 2021049/Ministry of Health and Care Services | Helse Sør-Øst RHF (Southern and Eastern Norway Regional Health Authority)
- 2020072/Ministry of Health and Care Services | Helse Sør-Øst RHF (Southern and Eastern Norway Regional Health Authority)
- 20240998/Ministry of Health and Care Services | Helse Sør-Øst RHF (Southern and Eastern Norway Regional Health Authority)
- 2022069/Ministry of Health and Care Services | Helse Sør-Øst RHF (Southern and Eastern Norway Regional Health Authority)
- 198091/Kreftforeningen (Norwegian Cancer Society)
- 190257/Kreftforeningen (Norwegian Cancer Society)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

