Effects of Exercise Training on Mitochondrial and Capillary Growth in Human Skeletal Muscle: A Systematic Review and Meta-Regression
- PMID: 39390310
- PMCID: PMC11787188
- DOI: 10.1007/s40279-024-02120-2
Effects of Exercise Training on Mitochondrial and Capillary Growth in Human Skeletal Muscle: A Systematic Review and Meta-Regression
Abstract
Background: Skeletal muscle mitochondria and capillaries are crucial for aerobic fitness, and suppressed levels are associated with chronic and age-related diseases. Currently, evidence-based exercise training recommendations to enhance these characteristics are limited. It is essential to explore how factors, such as fitness level, age, sex, and disease affect mitochondrial and capillary adaptations to different exercise stimuli.
Objectives: The main aim of this study was to compare the effects of low- or moderate intensity continuous endurance training (ET), high-intensity interval or continuous training (HIT), and sprint interval training (SIT) on changes in skeletal muscle mitochondrial content and capillarization. Secondarily, the effects on maximal oxygen consumption (VO2max), muscle fiber cross-sectional area, and fiber type proportion were investigated.
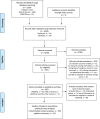
Methods: A systematic literature search was conducted in PubMed, Web of Science, and SPORTDiscus databases, with no data restrictions, up to 2 February 2022. Exercise training intervention studies of ET, HIT, and SIT were included if they had baseline and follow-up measures of at least one marker of mitochondrial content or capillarization. In total, data from 5973 participants in 353 and 131 research articles were included for the mitochondrial and capillary quantitative synthesis of this review, respectively. Additionally, measures of VO2max, muscle fiber cross-sectional area, and fiber type proportion were extracted from these studies.
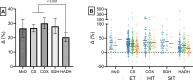
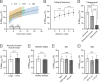
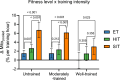
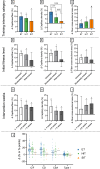
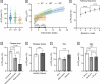
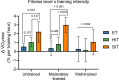
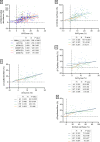
Results: After adjusting for relevant covariates, such as training frequency, number of intervention weeks, and initial fitness level, percentage increases in mitochondrial content in response to exercise training increased to a similar extent with ET (23 ± 5%), HIT (27 ± 5%), and SIT (27 ± 7%) (P > 0.138), and were not influenced by age, sex, menopause, disease, or the amount of muscle mass engaged. Higher training frequencies (6 > 4 > 2 sessions/week) were associated with larger increases in mitochondrial content. Per total hour of exercise, SIT was ~ 2.3 times more efficient in increasing mitochondrial content than HIT and ~ 3.9 times more efficient than ET, while HIT was ~ 1.7 times more efficient than ET. Capillaries per fiber increased similarly with ET (15 ± 3%), HIT (13 ± 4%) and SIT (10 ± 11%) (P = 0.556) after adjustments for number of intervention weeks and initial fitness level. Capillaries per mm2 only increased after ET (13 ± 3%) and HIT (7 ± 4%), with increases being larger after ET compared with HIT and SIT (P < 0.05). This difference coincided with increases in fiber cross-sectional area after ET (6.5 ± 3.5%), HIT (8.9 ± 4.9%), and SIT (11.9 ± 15.1%). Gains in capillarization occurred primarily in the early stages of training (< 4 weeks) and were only observed in untrained to moderately trained participants. The proportion of type I muscle fibers remained unaltered by exercise training (P > 0.116), but ET and SIT exhibited opposing effects (P = 0.041). VO2max increased similarly with ET, HIT, and SIT, although HIT showed a tendency for greater improvement compared with both ET and SIT (P = 0.082), while SIT displayed the largest increase per hour of exercise. Higher training frequencies (6 > 4 > 2 sessions/week) were associated with larger increases in VO2max. Women displayed greater percentage gains in VO2max compared with men (P = 0.008). Generally, lower initial fitness levels were associated with greater percentage improvements in mitochondrial content, capillarization, and VO2max. SIT was particularly effective in improving mitochondrial content and VO2max in the early stages of training, while ET and HIT showed slower but steady improvements over a greater number of training weeks.
Conclusions: The magnitude of change in mitochondrial content, capillarization, and VO2max to exercise training is largely determined by the initial fitness level, with greater changes observed in individuals with lower initial fitness. The ability to adapt to exercise training is maintained throughout life, irrespective of sex and presence of disease. While training load (volume × intensity) is a suitable predictor of changes in mitochondrial content and VO2max, this relationship is less clear for capillary adaptations.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Funding: No funding was received for this work. Conflict of interest: Knut Sindre Mølmen, Nicki Winfield Almquist, and Øyvind Skattebo declare that they have no conflicts of interest relevant to the content of this review. Ethics approval: Not applicable. Consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and material: The dataset, statistical analysis codes, and SAS scripts are available from the study’s GitHub repository. To request access, please contact the corresponding author with your GitHub username, and access will be granted upon reasonable request. Authors contribution: K.S.M. and Ø.S. conceived and designed the study, with input from N.W.A.; K.S.M. conducted the literature search and initial study selection; K.S.M. and N.W.A. performed research article screening and data extraction; Ø.S. did the statistical analyses and data visualization; K.S.M., N.W.A., and Ø.S. contributed to the interpretation of the findings; K.S.M. and Ø.S. wrote the first draft of the manuscript; All authors provided critical revision, feedback, comments, and input on the manuscript. All authors read and approved the final manuscript.
Figures

References
-
- Chepinoga O. Muscle tissue dehydrogenases in training and fatigue. Communication I: succinate dehydrogenase. Ukr Biochem J. 1939;XIV:5–14.
-
- Chepinoga O. Muscle tissue dehydrogenases in training and fatigue. Communication II: a-glycerophosphate dehydrogenase. Ukr Biochem J. 1939;XIV:15–29.
-
- Botella J, Motanova ES, Bishop DJ. Muscle contraction and mitochondrial biogenesis—a brief historical reappraisal. Acta Physiol. 2022;235:1–4. - PubMed
-
- Andersen P. Capillary density in skeletal muscle of man. Acta Physiol Scand [Internet]. 1975;95:203–5. http://www.ncbi.nlm.nih.gov/pubmed/127508. - PubMed
-
- Hood DA, Memme JM, Oliveira AN, Triolo M. Maintenance of skeletal muscle mitochondria in health, exercise, and aging. Annu Rev Physiol [Internet]. 2019;81:19–41. http://www.ncbi.nlm.nih.gov/pubmed/30216742. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

