Selective neuronal expression of progranulin is sufficient to provide neuroprotective and anti-inflammatory effects after traumatic brain injury
- PMID: 39390556
- PMCID: PMC11468377
- DOI: 10.1186/s12974-024-03249-7
Selective neuronal expression of progranulin is sufficient to provide neuroprotective and anti-inflammatory effects after traumatic brain injury
Abstract
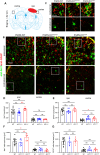
Progranulin (PGRN), which is produced in neurons and microglia, is a neurotrophic and anti-inflammatory glycoprotein. Human loss-of-function mutations cause frontotemporal dementia, and PGRN knockout (KO) mice are a model for dementia. In addition, PGRN KO mice exhibit severe phenotypes in models of traumatic or ischemic central nervous system (CNS) disorders, including traumatic brain injury (TBI). It is unknown whether restoration of progranulin expression in neurons (and not in microglia) might be sufficient to prevent excessive TBI-evoked brain damage. To address this question, we generated mice with Nestin-Cre-driven murine PGRN expression in a PGRN KO line (PGRN-KONestinGrn) to rescue PGRN in neurons. PGRN expression analysis in primary CNS cell cultures from naïve mice and in (non-) injured brain tissue from PGRN-KONestinGrn revealed expression of PGRN in neurons but not in microglia. After experimental TBI, examination of the structural brain damage at 5 days post-injury (dpi) showed that the TBI-induced loss of brain tissue and hippocampal neurons was exacerbated in PGRN-KOGrnflfl mice (PGRN knockout with the mGrn fl-STOP-fl allele, Cre-negative), as expected, whereas the tissue damage in PGRN-KONestinGrn mice was similar to that in PGRN-WT mice. Analysis of CD68+ immunofluorescent microglia and Cd68 mRNA expression showed that excessive microglial activation was rescued in PGRN-KONestinGrn mice, and the correlation of brain injury with Cd68 expression suggested that Cd68 was a surrogate marker for excessive brain injury caused by PGRN deficiency. The results show that restoring neuronal PGRN expression was sufficient to rescue the exacerbated neuropathology of TBI caused by PGRN deficiency, even in the absence of microglial PGRN. Hence, endogenous microglial PGRN expression was not essential for the neuroprotective or anti-inflammatory effects of PGRN after TBI in this study.
Keywords: CD68; Microglia; Neuroinflammation; Neuropathology; Neuroprotection; Progranulin; Therapy; Traumatic brain injury.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Petkau TL, Neal SJ, Orban PC, MacDonald JL, Hill AM, Lu G, et al. Progranulin expression in the developing and adult murine brain. J Comp Neurol. 2010;518(19):3931–47. - PubMed
-
- Thomasen PB, Salasova A, Kjaer-Sorensen K, Woloszczuková L, Lavický J, Login H, et al. SorCS2 binds progranulin to regulate motor neuron development. Cell Rep. 2023;42(11):113333. - PubMed
-
- Altmann C, Hardt S, Fischer C, Heidler J, Lim H-Y, Häussler A, et al. Progranulin overexpression in sensory neurons attenuates neuropathic pain in mice: role of autophagy. Neurobiol Dis. 2016;96:294–311. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

