Consensus, debate, and prospective on pancreatic cancer treatments
- PMID: 39390609
- PMCID: PMC11468220
- DOI: 10.1186/s13045-024-01613-x
Consensus, debate, and prospective on pancreatic cancer treatments
Abstract
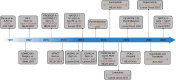
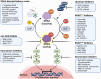
Pancreatic cancer remains one of the most aggressive solid tumors. As a systemic disease, despite the improvement of multi-modality treatment strategies, the prognosis of pancreatic cancer was not improved dramatically. For resectable or borderline resectable patients, the surgical strategy centered on improving R0 resection rate is consensus; however, the role of neoadjuvant therapy in resectable patients and the optimal neoadjuvant therapy of chemotherapy with or without radiotherapy in borderline resectable patients were debated. Postoperative adjuvant chemotherapy of gemcitabine/capecitabine or mFOLFIRINOX is recommended regardless of the margin status. Chemotherapy as the first-line treatment strategy for advanced or metastatic patients included FOLFIRINOX, gemcitabine/nab-paclitaxel, or NALIRIFOX regimens whereas 5-FU plus liposomal irinotecan was the only standard of care second-line therapy. Immunotherapy is an innovative therapy although anti-PD-1 antibody is currently the only agent approved by for MSI-H, dMMR, or TMB-high solid tumors, which represent a very small subset of pancreatic cancers. Combination strategies to increase the immunogenicity and to overcome the immunosuppressive tumor microenvironment may sensitize pancreatic cancer to immunotherapy. Targeted therapies represented by PARP and KRAS inhibitors are also under investigation, showing benefits in improving progression-free survival and objective response rate. This review discusses the current treatment modalities and highlights innovative therapies for pancreatic cancer.
Keywords: Chemotherapy; Immunotherapy; Pancreatic cancer; Radiotherapy; Surgery; Targeted therapy; Tumor microenvironment.
© 2024. The Author(s).
Conflict of interest statement
L.Z. receives grant support from Bristol-Meyer Squibb, Merck, AstraZeneca, iTeos, Amgen, NovaRock, Inxmed, Halozyme and Abmeta. L.Z. is a paid consultant/Advisory Board Member at Biosion, Alphamab, NovaRock, Ambrx, Akrevia/Xilio, QED, Novagenesis, Snow Lake Capitals, Amberstone, Pfizer, Tavotek, and Mingruizhiyao. L.Z. holds shares at Alphamab, Amberstone, Mingruizhiyao, and Cellaration. LZ is an editorial board member of Journal of Hematology and Oncology.
Figures

References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17–48. - PubMed
-
- Kotecha K, Tree K, Ziaziaris WA, McKay SC, Wand H, Samra J, et al. Centralization of pancreaticoduodenectomy: a systematic review and spline regression analysis to recommend minimum volume for a specialist pancreas service. Ann Surg. 2024;279(6):953–60. - PubMed
-
- Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Comprehens Cancer Netw JNCCN. 2017;15(8):1028–61. - PubMed
Publication types
MeSH terms
Grants and funding
- 82303740/National Natural Science Foundation of China
- 2023YFS0167/Science and Technology Department of Sichuan Province
- P30 CA006973/CA/NCI NIH HHS/United States
- P01 CA247886/CA/NCI NIH HHS/United States
- P01 CA247886/NH/NIH HHS/United States
- R01 CA197296/NH/NIH HHS/United States
- R01 CA169702/NH/NIH HHS/United States
- 82403318/National Natural Science Foundation of China
- 2024HXBH134/Postdoctor Research Fund of West China Hospital, Sichuan University
- P30 CA006973./NH/NIH HHS/United States
- 2023T160451/China Postdoctoral Science Foundation
- 2023HXBH053/Postdoctor Research Fund of West China Hospital, Sichuan University
- 2024NSFSC1949/Science and Technology Department of Sichuan Province
- P50 CA062924/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

