Asia-Pacific consensus recommendations on the management of generalized pustular psoriasis
- PMID: 39390737
- PMCID: PMC11624156
- DOI: 10.1111/1346-8138.17471
Asia-Pacific consensus recommendations on the management of generalized pustular psoriasis
Abstract
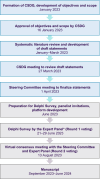
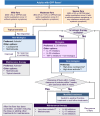
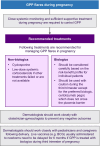
Generalized pustular psoriasis (GPP) is a rare, chronic, heterogeneous, and potentially life-threatening disease characterized by primary, sterile, and macroscopically visible pustules with or without systemic symptoms. There are ethnic differences in the genetic mutations associated with GPP that might affect the clinical manifestations and treatment responses. Currently, there is limited evidence from the patient population in the Asia-Pacific (APAC) region, resulting in a general paucity of information on the effective management of patients with GPP in this region. This modified Delphi panel study aimed to identify current evidence and gain advanced insights to facilitate the development of a regionally tailored APAC consensus on the management of GPP. A systematic literature review (SLR) was conducted to identify published literature and develop consensus statements on (i) definition and clinical course, (ii) diagnosis of GPP, (iii) treatment outcomes, goals, and monitoring measures, and (iv) optimal management strategies and clinical practices. Statements were rated by a panel of dermatologists in two rounds, with the threshold for consensus at ≥80% agreement. Twenty experts from the APAC region reached consensus on 106 statements that were developed based on the SLR and experts' collective expertise. The experts agreed that GPP is a rare, severe, and potentially life-threatening condition that is distinct from plaque psoriasis. This consensus emphasized the importance of a tailored treatment strategy taking into account the GPP flare severity and each patient's unique clinical circumstances. The experts reached consensus on the severity classification of GPP flares and recommended first-line and maintenance treatment options for adult GPP, childhood GPP, and GPP in pregnancy. These consensus outcomes have been synthesized into treatment algorithms to guide dermatologists in the APAC region in their clinical decision-making processes.
Keywords: Asia‐Pacific; Delphi panel; consensus; generalized pustular psoriasis; management.
© 2024 The Author(s). The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
S.E.C. declared paid activities as an advisor, speaker or consultant for AbbVie, Almirall, Boehringer Ingelheim, Eli Lilly, Janssen, Novartis, and Pfizer. P.A.F. declared receiving grant support from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Sanofi, Sun Pharma, and UCB; travel grants from AbbVie, Eli Lilly, Galderma, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Roche, Sanofi, and Sun Pharma; and declared activities as an investigator for AbbVie, Amgen, Arcutis, Argenx, ASLAN, AstraZeneca, Boehringer Ingelheim, Botanix, Bristol Myers Squibb, Celgene, Celtaxsys, CSL, Cutanea, Dermira, Eli Lilly, Evelo Biosciences, Galderma, Genentech, Geneseq, GlaxoSmithKline, Hexima, Incyte, Janssen, Kymab, Leo Pharma, Merck, Novartis, Pfizer, Regeneron, Reistone, Roche, Sanofi, Sun Pharma, Takeda, Teva, UCB, Valeant, and ZaiLab; an advisory board member for AbbVie, Amgen, Aslan, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, Leo Pharma, Mayne Pharma, Novartis, Pfizer, Sanofi, Sun Pharma, UCB, and Valeant; a consultant for Aslan, Bristol Myers Squibb, Eli Lilly, Galderma, GenesisCare, Hexima, Janssen, Leo Pharma, Mayne Pharma, MedImmune, Novartis, Pfizer, Roche, and UCB; as a speaker or honoraria recipient from AbbVie, Amgen, Bristol Myers Squibb, Celgene, Eli Lilly, Galderma, GlaxoSmithKline, Janssen, Leo Pharma, Merck, Novartis, Pfizer, Roche, Sanofi, Sun Pharma, and Valeant. P.A. declared receiving honoraria or payment from Boehringer Ingelheim, Pfizer, Eli Lilly, Novartis and Janssen, and support for attending meetings and/or travel from Pfizer, Eli Lilly, and Novartis. H.F. has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant, as well as grants as an investigator from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eisai, Eli Lilly, Janssen, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi‐Tanabe, Novartis, Sanofi, Sun Pharma, Taiho, Torii, UCB, and Ushio. H.F. is an Editorial Board member of
Hideki Fujita is an Editorial Board member of
Figures


References
-
- Choon SE, Lai NM, Mohammad NA, Nanu NM, Tey KE, Chew SF. Clinical profile, morbidity, and outcome of adult‐onset generalized pustular psoriasis: analysis of 102 cases seen in a tertiary hospital in Johor, Malaysia. Int J Dermatol. 2014;53:676–684. - PubMed
-
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15:907–919. - PubMed
-
- Navarini AA, Burden AD, Capon F, Mrowietz U, Puig L, Koks S, et al. European consensus statement on phenotypes of pustular psoriasis. J Eur Acad Dermatol Venereol. 2017;31:1792–1799. - PubMed
-
- Puig L, Choon SE, Gottlieb AB, Marrakchi S, Prinz JC, Romiti R, et al. Generalized pustular psoriasis: a global Delphi consensus on clinical course, diagnosis, treatment goals and disease management. J Eur Acad Dermatol Venereol. 2023;37:737–752. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

