Population pharmacokinetics of tacrolimus whole blood and peripheral blood mononuclear cell concentrations in stable kidney-transplanted patients
- PMID: 39390741
- PMCID: PMC11862786
- DOI: 10.1111/bcp.16277
Population pharmacokinetics of tacrolimus whole blood and peripheral blood mononuclear cell concentrations in stable kidney-transplanted patients
Abstract
Aim: Therapeutic drug monitoring of tacrolimus based on whole blood drug concentrations is routinely performed. The concentration of tacrolimus in peripheral blood mononuclear cells (PMBCs) is likely to better reflect drug exposure at the treatment target site. We aimed to describe the relationship between tacrolimus whole blood and PBMC concentrations, and the influence of patient characteristics on this relationship by developing a population pharmacokinetic model.
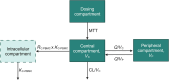
Methods: We prospectively enrolled 63 stable adult kidney-transplanted patients and collected dense (12-h, n = 18) or sparse (4-h, n = 45) pharmacokinetic profiles of tacrolimus. PBMCs were isolated from whole blood (Ficoll density gradient centrifugation), and drug concentrations in whole blood and PBMCs were analysed using liquid chromatography-mass spectrometry. Patient genotype (CYP3A4/5, ABCB1, NR1I2) was assessed with PCR. Population pharmacokinetic modelling and statistical evaluation was performed using NONMEM.
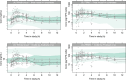
Results: Tacrolimus whole blood concentrations were well described using a two-compartment pharmacokinetic model with a lag-time and first-order absorption and elimination. Tacrolimus PBMC concentrations were best estimated from whole blood concentrations with the use of a scaling factor, the ratio of whole blood to PBMC concentrations (RC:PBMC), which was the extent of tacrolimus distribution into PBMC. CYP3A5*1 non-expressors and NR1I2-25 385T allele expressors demonstrated higher RC:PBMC ratios of 42.4% and 60.7%, respectively.
Conclusion: Tacrolimus PBMC concentration could not be accurately predicted from whole blood concentrations and covariates because of significant residual unexplained variability in the distribution of tacrolimus into PBMCs and may need to be measured directly if required for future studies.
Keywords: immunosuppressants; pharmacogenetics; population pharmacokinetics; therapeutic drug monitoring; transplantation.
© 2024 The Author(s). British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
T.B.S. has given paid lectures for Pfizer and Eisai and done consulting for Pfizer, and collaborated with Novo Nordisk A/S, all unrelated to the work reported in the present paper. All other authors declare no conflict of interests.
Figures



References
-
- Kuypers DR, Claes K, Evenepoel P, Maes B, Vanrenterghem Y. Clinical efficacy and toxicity profile of tacrolimus and mycophenolic acid in relation to combined long‐term pharmacokinetics in de novo renal allograft recipients. Clin Pharmacol Ther. 2004;75(5):434‐447. doi:10.1016/j.clpt.2003.12.009 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

