Bactericidal and anti-quorum sensing activity of repurposing drug Visomitin against Staphylococcus aureus
- PMID: 39390774
- PMCID: PMC11492638
- DOI: 10.1080/21505594.2024.2415952
Bactericidal and anti-quorum sensing activity of repurposing drug Visomitin against Staphylococcus aureus
Abstract
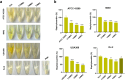
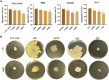
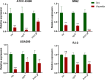
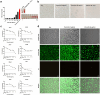
With the growing antibiotic resistance in Staphylococcus aureus, it is imperative to develop innovative therapeutic strategies against new targets to reduce selective survival pressures and incidence of resistance. In S. aureus, interbacterial communication relies on a quorum sensing system that regulates gene expression and physiological activities. Here, we identified that Visomitin, an antioxidant small molecule, exhibited bactericidal efficacy against methicillin-resistant S. aureus and its high tolerance phenotypes like intracellular bacteria and persister cells without inducing resistance. Critically, sub-minimal inhibitory concentrations (sub-MICs) of Visomitin could serve as a potent quorum-quencher reducing virulence production (such as haemolysin and staphyloxanthin), along with inhibiting biofilm formation, self-aggregation, and colony spreading of S. aureus. These effects were probably mediated by interfering with the S. aureus accessory gene regulator quorum sensing system. In summary, our findings suggest that Visomitin shows dual antimicrobial effects, including bactericidal effects at the concentrations above MIC and quorum sensing inhibition effects at sub-MICs, which holds promise for treating MRSA-related refractory infections.
Keywords: Methicillin-resistant Staphylococcus aureus; Visomitin; antimicrobial; biofilm; haemolysis; quorum sensing.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
