Modeling respiratory tract diseases for clinical translation employing conditionally reprogrammed cells
- PMID: 39391007
- PMCID: PMC11462205
- DOI: 10.1016/j.cellin.2024.100201
Modeling respiratory tract diseases for clinical translation employing conditionally reprogrammed cells
Abstract
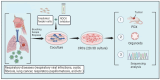
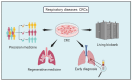
Preclinical models serve as indispensable tools in translational medicine. Specifically, patient-derived models such as patient-derived xenografts (PDX), induced pluripotent stem cells (iPSC), organoids, and recently developed technique of conditional reprogramming (CR) have been employed to reflect the host characteristics of diseases. CR technology involves co-culturing epithelial cells with irradiated Swiss-3T3-J2 mouse fibroblasts (feeder cells) in the presence of a Rho kinase (ROCK) inhibitor, Y-27632. CR technique facilitates the rapid conversion of both normal and malignant cells into a "reprogrammed stem-like" state, marked by robust in vitro proliferation. This is achieved without reliance on exogenous gene expression or viral transfection, while maintaining the genetic profile of the parental cells. So far, CR technology has been used to study biology of diseases, targeted therapies (precision medicine), regenerative medicine, and noninvasive diagnosis and surveillance. Respiratory diseases, ranking as the third leading cause of global mortality, pose a significant burden to healthcare systems worldwide. Given the substantial mortality and morbidity rates of respiratory diseases, efficient and rapid preclinical models are imperative to accurately recapitulate the diverse spectrum of respiratory conditions. In this article, we discuss the applications and future potential of CR technology in modeling various respiratory tract diseases, including lung cancer, respiratory viral infections (such as influenza and Covid-19 and etc.), asthma, cystic fibrosis, respiratory papillomatosis, and upper aerodigestive track tumors. Furthermore, we discuss the potential utility of CR in personalized medicine, regenerative medicine, and clinical translation.
Keywords: Asthma; Conditional reprogramming; Covid-19; Cystic fibrosis; Lung cancer; Preclinical models; Respiratory diseases; Respiratory papillomatosis; Respiratory viral infections; Translational medicine.
© 2024 The Author(s).
Conflict of interest statement
Several patents for CR technology have been awarded to Georgetown University by the US Patent Office. The license for this technology has been given to a Maryland-based start-up company for commercialization. The inventor, X.L., and Georgetown University receive potential royalties and payments from the company. CR media and CR cells have been distributed by Propagenix (acquired by StemCell Technologies), Fisher Scientific, ATCC, etc. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Awatade N.T., et al. Significant functional differences in differentiated Conditionally Reprogrammed (CRC)-and Feeder-free Dual SMAD inhibited-expanded human nasal epithelial cells. Journal of Cystic Fibrosis. 2021;20(2):364–371. - PubMed
-
- Bear C.E. A therapy for most with cystic fibrosis. Cell. 2020;180(2):211. - PubMed
-
- Belinsky S.A. Gene-promoter hypermethylation as a biomarker in lung cancer. Nature Reviews Cancer. 2004;4(9):707–717. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

