Single-cell transcriptomic analysis of flowering regulation and vernalization in Chinese cabbage shoot apex
- PMID: 39391013
- PMCID: PMC11464683
- DOI: 10.1093/hr/uhae214
Single-cell transcriptomic analysis of flowering regulation and vernalization in Chinese cabbage shoot apex
Abstract
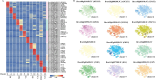
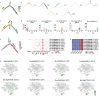
In Chinese cabbage development the interplay between shoot apex activity and vernalization is pivotal for flowering timing. The intricate relationship between various cell types in the shoot apex meristem and their roles in regulating flowering gene expression in Chinese cabbage is not yet fully understood. A thorough analysis of single-cell types in the Chinese cabbage shoot apex and their influence on flowering genes and vernalization is essential for deeper insight. Our study first established a single-cell transcriptomic atlas of Chinese cabbage after 25 days of non-vernalization. Analyzing 19 602 single cells, we differentiated them into 15 distinct cell clusters using established marker genes. We found that key genes in shoot apex development and flowering were primarily present in shoot meristematic cells (SMCs), companion cells (CCs), and mesophyll cells (MCs). MADS-box protein FLOWERING LOCUS C 2 (BrFLC2), a gene suppressing flowering, was observed in CCs, mirroring patterns found in Arabidopsis. By mapping developmental trajectories of SMCs, CCs, and MCs, we elucidated the evolutionary pathways of crucial genes in shoot apex development and flowering. The creation of a single-cell transcriptional atlas of the Chinese cabbage shoot apex under vernalization revealed distinct alterations in the expression of known flowering genes, such as VERNALIZATION INSENSITIVE 3 (VIN3), VERNALIZATION 1 (VRN1), VERNALIZATION 2 (VRN2), BrFLC, and FLOWERING LOCUS T (FT), which varied by cell type. Our study underscores the transformative impact of single-cell RNA sequencing (scRNA-seq) for unraveling the complex differentiation and vernalization processes in the Chinese cabbage shoot apex. These insights are pivotal for enhancing breeding strategies and cultivation management of this vital vegetable.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nanjing Agricultural University.
Conflict of interest statement
The authors confirm that they have no conflicts of interest concerning this research.
Figures





References
-
- Luo X, He YH. Experiencing winter for spring flowering: a molecular epigenetic perspective on vernalization. J Integr Plant Biol. 2020;62:104–17 - PubMed
-
- Levy YY, Mesnage S, Mylne JS. et al. Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science. 2002;297:243–6 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

