Inactivation of Tnf-α/Tnfr signaling attenuates progression of intervertebral disc degeneration in mice
- PMID: 39391171
- PMCID: PMC11461905
- DOI: 10.1002/jsp2.70006
Inactivation of Tnf-α/Tnfr signaling attenuates progression of intervertebral disc degeneration in mice
Abstract
Background: Intervertebral disc degeneration (IVDD) is a major cause of low back pain (LBP), worsened by chronic inflammatory processes associated with aging. Tumor necrosis factor alpha (Tnf-α) and its receptors, Tnf receptor type 1 (Tnfr1) and Tnf receptor type 2 (Tnfr2), are upregulated in IVDD. However, its pathologic mechanisms remain poorly defined.
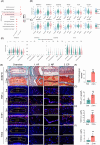
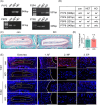
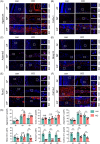
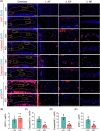
Methods: To investigate the role of Tnfr in IVDD, we generated global Tnfr1/2 double knockout (KO) mice and age-matched control C57BL/6 male mice, and analyzed intervertebral disc (IVD)-related phenotypes of both genotypes under physiological conditions, aging, and lumbar spine instability (LSI) model through histological and immunofluorescence analyses and μCT imaging. Expression levels of key extracellular matrix (ECM) proteins in aged and LSI mice, especially markers of cell proliferation and apoptosis, were evaluated in aged (21-month-old) mice.
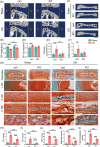
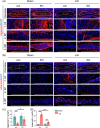
Results: At 4 months, KO and control mice showed no marked differences of IVDD-related parameters. However, at 21 months of age, the loss of Tnfr expression significantly alleviated IVDD-like phenotypes, including a significant increase in height of the nucleus pulposus (NPs) and reductions of endplates (EPs) porosity and histopathological scores, when compared to controls. Tnfr deficiency promoted anabolic metabolism of the ECM proteins and suppressed ECM catabolism. Tnfr loss largely inhibited hypertrophic differentiation, and, in the meantime, suppressed cell apoptosis and cellular senescence in the annulus fibrosis, NP, and EP tissues without affecting cell proliferation. Similar results were observed in the LSI model, where Tnfr deficiency significantly alleviated IVDD and enhanced ECM anabolic metabolism while suppressing catabolism.
Conclusion: The deletion of Tnfr mitigates age-related and LSI-induced IVDD, as evidenced by preserved IVD structure, and improved ECM integrity. These findings suggest a crucial role of Tnf-α/Tnfr signaling in IVDD pathogenesis in mice. Targeting this pathway may be a novel strategy for IVDD prevention and treatment.
Keywords: TNF receptor; intervertebral disc degeneration; tumor necrosis factor‐α.
© 2024 The Author(s). JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures

Similar articles
-
Distinctive roles of tumor necrosis factor receptor type 1 and type 2 in a mouse disc degeneration model.J Orthop Translat. 2021 Dec 3;31:62-72. doi: 10.1016/j.jot.2021.11.003. eCollection 2021 Nov. J Orthop Translat. 2021. PMID: 34934623 Free PMC article.
-
TNFR1-mediated senescence and lack of TNFR2-signaling limit human intervertebral disc cell repair potential in degenerative conditions.Osteoarthritis Cartilage. 2025 Jul;33(7):874-887. doi: 10.1016/j.joca.2025.02.791. Epub 2025 Mar 24. Osteoarthritis Cartilage. 2025. PMID: 40139648
-
Ginsenoside Rg1 relieves rat intervertebral disc degeneration and inhibits IL-1β-induced nucleus pulposus cell apoptosis and inflammation via NF-κB signaling pathway.In Vitro Cell Dev Biol Anim. 2024 Mar;60(3):287-299. doi: 10.1007/s11626-024-00883-6. Epub 2024 Mar 14. In Vitro Cell Dev Biol Anim. 2024. PMID: 38485818 Free PMC article.
-
IGF Signaling in Intervertebral Disc Health and Disease.Front Cell Dev Biol. 2022 Feb 1;9:817099. doi: 10.3389/fcell.2021.817099. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35178405 Free PMC article. Review.
-
The pathological mechanisms of circRNAs in mediating intervertebral disc degeneration.Noncoding RNA Res. 2023 Sep 18;8(4):633-640. doi: 10.1016/j.ncrna.2023.09.004. eCollection 2023 Dec. Noncoding RNA Res. 2023. PMID: 37780894 Free PMC article. Review.
Cited by
-
Combined Oxygen-Ozone and Porcine Injectable Collagen Therapies Boosting Efficacy in Low Back Pain and Disability.Diagnostics (Basel). 2024 Oct 29;14(21):2411. doi: 10.3390/diagnostics14212411. Diagnostics (Basel). 2024. PMID: 39518378 Free PMC article.
-
A Novel Approach to Predict the Location and Fatigue Life of Intervertebral Disc Degeneration.Bioengineering (Basel). 2025 Apr 16;12(4):423. doi: 10.3390/bioengineering12040423. Bioengineering (Basel). 2025. PMID: 40281783 Free PMC article.
References
-
- Balagué F, Mannion AF, Pellisé F, Cedraschi C. Non‐specific low back pain. Lancet. 2012;379(9814):482‐491. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

