A roadmap to cure CHD2-related disorders
- PMID: 39391213
- PMCID: PMC11465304
- DOI: 10.1177/26330040241283749
A roadmap to cure CHD2-related disorders
Abstract
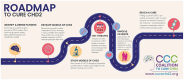
Coalition to Cure CHD2 (CCC) is a patient advocacy group dedicated to improving the lives of those affected by CHD2-related disorders (CHD2-RD) by increasing education, building community, and accelerating research to uncover a cure. CHD2 is a chromatin remodeler that was identified in 2013 as being a genetic cause for developmental and epileptic encephalopathies. Pathogenic changes in CHD2 can cause treatment-resistant epilepsy, intellectual and developmental delays, and autism, and some individuals experience neurodevelopmental regression. There are currently no targeted therapies available for CHD2-related disorders. Haploinsufficiency of CHD2 is a causative mechanism of disease for individuals with pathogenic variants (primarily truncating) in CHD2. Recently, identification of individuals with deletion of nearby gene CHASERR, a regulator of CHD2 gene expression, has established dosage sensitivity in CHD2 and solidified the CHASERR gene as a potential therapeutic target for CHD2 levels. Through collaboration with our community and our scientific advisory board, CCC has created a Roadmap to Cure CHD2 as our guide toward a targeted cure that can benefit our community, with steps including (1) identifying and defining patients, (2) developing models of CHD2, (3) studying models of CHD2, (4) testing therapies, (5) involving patients, and (6) reaching a cure. Despite some of the challenges inherent in CHD2 research including establishing animal and cellular models that recapitulate the CHD2 clinical phenotype, identifying measurable outcomes and reliable biomarkers, or testing emerging therapeutic approaches, CCC continues to engage with our community to support ongoing research that aligns with our priorities. CCC sees new and exciting opportunities for additional research that can move our community toward our common goal of a cure that will improve the lives of individuals and their families now and in the future.
Keywords: CHASERR; CHD2; DNA Methylation; Developmental and Epileptic Encephalopathy (DEE); epilepsy; long noncoding RNAs; rare disease.
Plain language summary
A roadmap to cure disorders caused by the CHD2 and CHASERR genes Coalition to Cure CHD2 (CCC) is a nonprofit founded in October 2020 to fund research towards a cure for individuals with CHD2-related disorders. The CHD2 gene was discovered as a genetic cause for epilepsy in 2013. Individuals with CHD2 typically experience seizures that can be resistant to treatment, intellectual disability, delayed development, autism, and other symptoms. The nearby CHASERR gene has been found to regulate CHD2 and is a possible therapeutic target. Individuals with a deletion of CHASERR have been identified - these individuals have too much CHD2 and more severe symptoms. CCC has created a Roadmap to Cure CHD2 as a guide for their journey towards a targeted cure for CHD2-related disorders. The steps in the roadmap include: (1) identify and define patients, (2) develop models of CHD2, (3) study models of CHD2, (4) test therapies, (5) involve patients, (6) reach a cure. CCC has worked with CHD2 families to identify family-level priorities for therapeutic development (e.g. seizures, behavior, etc), to capture the impact of disease through qualitative research, and to collect patient health data and tissue samples for scientific analysis. The development of CHD2 models, mouse models in particular, has been challenging as the mice do not develop seizures. Additional models are underway including frogs, zebrafish, and patient-derived cells. These models have provided crucial insight into the biology of CHD2 but scientific questions remain unanswered. A variety of therapeutic approaches have been proposed including novel treatments that directly target CHD2 biology as well as the repurposing of existing FDA-approved compounds. Establishing measurable outcomes, including biomarkers, and finding treatments that can reach the brain will be important. By continuing to follow this roadmap, the CCC believes that one day there will be a cure for CHD2-related disorders.
© The Author(s), 2024.
Conflict of interest statement
C.M., H.C.M., and G.C. serve of the Scientific Advisory Board of Coalition to Cure CHD2. S.P., C.S., and B.B. serve on the Board of Directors of Coalition To Cure CHD2. B.B. is Co-President of Coalition To Cure CHD2.
Figures




References
-
- Carvill GL, Mefford HC. CHD2-Related Neurodevelopmental Disorders. In: Adam MP, Feldman J, Mrizaa GM, et al.. (eds) GeneReviews® [Internet], Updated 2021. Seattle: University of Washington, 1993; 2015.
-
- Ganesh VS, Riquin K, Chatron N, et al.. Novel syndromic neurodevelopmental disorder caused by de novo deletion of CHASERR, a long noncoding RNA. medRxiv. Epub ahead of print 7 February 2024. DOI: 10.1101/2024.01.31.24301497 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
