A roadmap for improving representation in clinical trials
- PMID: 39391227
- PMCID: PMC11466549
- DOI: 10.1016/j.conctc.2024.101374
A roadmap for improving representation in clinical trials
Abstract
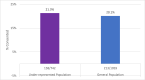
Clinical trials continue to struggle with recruiting diverse participants that include historically underrepresented and minoritized patients, who are typically patients in non-white racial and ethnic groups and have low income (Medicaid). Enrolling diverse participants will benefit the health sciences by providing more generalizable findings. The Cancer Financial Experience project (CAFÉ) study sought to improve financial distress by providing financial navigation for newly diagnosed cancer patients, and intentionally recruited diverse participants. All diverse participants consented at slightly higher rates than non-diverse participants (21.3 % vs. 20.1 %). Spanish-speaking patients consented at a much higher rate than non-Spanish speakers (36.4 % vs. 20.2 % respectively). Here we discuss how we increased our recruitment of diverse participants. Obtaining diverse participation is achievable and will provide more meaningful findings.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nora Henrickson and Mateo Banegas report financial support for this project was provided by 10.13039/100000054National Cancer Institute. Gloria Coronado reports a relationship with Exact Sciences Corporation that includes: consulting or advisory. Other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- National Institutes of Health Inclusion policies for research involving human subjects. 2024. https://grants.nih.gov/policy/inclusion.htm NIH.
-
- U.S. Food and Drug Administration 2019 Drug trials snapshots summary report: five-year summary and analysis of clinical trial participation and demographics. Five-year summary and analysis of clinical trial participation and demographics. 2015. https://www.fda.gov/media/143592/download
-
- National Academies of Sciences E, Medicine, Policy, et al. In: Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Bibbins-Domingo K., Helman A., editors. National Academies Press (US) Copyright 2022 by the National Academy of Sciences. All rights reserved.; 2022. The national Academies collection: reports funded by national institutes of health. - PubMed
LinkOut - more resources
Full Text Sources

