Copper catalyzed benzylic sp3 C-H alkenylation
- PMID: 39391381
- PMCID: PMC11459437
- DOI: 10.1039/d4sc03430a
Copper catalyzed benzylic sp3 C-H alkenylation
Abstract
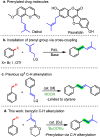
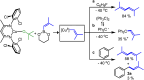
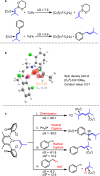
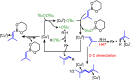
The prenyl group is present in numerous biologically active small molecule drugs and natural products. We introduce benzylic C-H alkenylation of substrates Ar-CH3 with alkenylboronic esters (CH2)3O2B-CH[double bond, length as m-dash]CMe2 as a pathway to form prenyl functionalized arenes Ar-CH2CH[double bond, length as m-dash]CMe2. Mechanistic studies of this radical relay catalytic protocol reveal diverse reactivity pathways exhibited by the copper(ii) alkenyl intermediate [CuII]-CH[double bond, length as m-dash]CMe2 that involve radical capture, bimolecular C-C bond formation, and hydrogen atom transfer (HAT).
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

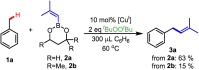

References
-
- Mosquera M. E. G. Jiménez G. Tabernero V. Vinueza-Vaca J. García-Estrada C. Kosalková K. Sola-Landa A. Monje B. Acosta C. Alonso R. Valera M. Á. Sustainable Chem. 2021;2:467–492. doi: 10.3390/suschem2030026. - DOI
-
- Šmejkal K. Phytochem. Rev. 2014;13:245–275. doi: 10.1007/s11101-013-9308-2. - DOI
-
- Yang X. Jiang Y. Yang J. He J. Sun J. Chen F. Zhang M. Yang B. Trends Food Sci. Technol. 2015;44:93–104. doi: 10.1016/j.tifs.2015.03.007. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

